The nuclear envelope consists of an outer membrane, an inner membrane, a perinuclear space between them, and a nuclear lamina. At some points, the inner and the outer membranes are continuous in a way that form open passages that make possible a direct communication between cytoplasm and nucleoplasm. Nuclear pore complexes are macromolecular structures located in these passages of the nuclear envelope. They are very large protein aggregates, so big that they can be observed with the electron microscope (Figure 1). They are the communication gates between nucleoplasm and cytoplasm, since all the molecular trafficking between these two compartments occurs through the nuclear pore complexes. Controlling this traffic is vital for the cell. For instance, they are key elements during the cell response to external stimuli and during cell differentiation. The entrance of transcription factors into the nucleus influences the expression of particular genes, and the exit of mRNA makes it possible the synthesis of proteins.
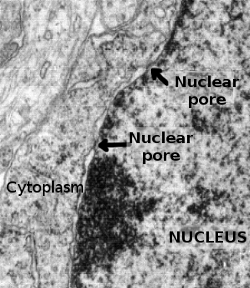
Nuclear pore complexes are very abundant in cells showing an intense trafficking between nucleoplasm and cytoplasm, as in differentiating cells. Around 11 nuclear pore complexes per μm2 of nuclear envelope are estimated, which means around 3000 to 4000 nuclear pores in a single cell. During the cell cycle, new nuclear pore complexes are synthesized and assembled in interphase, before mitosis. However, they are also synthesized after cell division. In open mitosis, where the nuclear envelope is disorganized, the nuclear pore complexes are also disassembled, and their proteins are freed in the cytoplasm. After mitosis, these nuclear pore proteins are assembled again in new nuclear pore complexes while the nuclear envelope is organized at the same time.
1. Components
Proteins of the nuclear pore complexes are known as nucleoporins. In yeasts, 30 different nucleoporins have been found, whereas in metazoa may be more than 40 different nucleoporins. There are several copies of each nucleoporin in the same nuclear pore complex. Thus, in mammals, a nuclear pore complex contains around 500 to 1000 nucleoporins. The total structure is 100 to 150 nm wide, 50 to 70 nm high, and contains an inner hydrophilic passage of around 40 nm. Nuclear pore complexes are among the largest protein structures of the cell, about 125000 kDa. It is remarkable that nucleoporins show a great stability, sometimes as long as the whole life of the cell, whereas the lifetime of a common protein may last a few days.
Nucleoporins are grouped in 8 blocks, which are organized as a regular octagon forming ring-like structures (Figure 2). The cytoplasmic ring faces the cytoplasm, the radial ring is in the channel of the nuclear envelope and anchors the nuclear pore complex to the nuclear envelope, and the nuclear ring is facing the nucleoplasm. Furthermore, there are fibrils extending from each of the 8 blocks: cytoplasmic fibrils and intranuclear fibrils. The intranuclear fibrils are connected to the nuclear ring by one of their ends and to other proteins that form another intranuclear ring known as the distal ring. Intranuclear fibrils and distal ring together form the nuclear basket, also known as the nuclear cage.
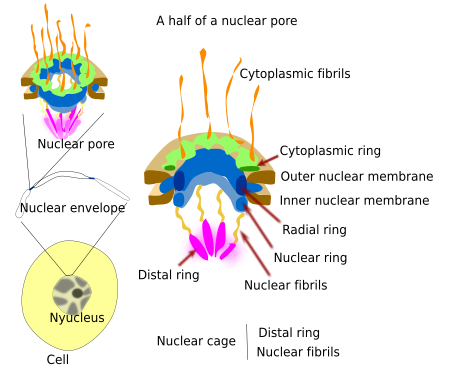
Besides the structural role, nucleoporins are classified according to their function. There are transmembrane proteins for anchoring the whole complex to the membrane of the nuclear envelope. The scaffold proteins form the rings, and the inner proteins form the hydrophilic passage and regulate the molecular trafficking through the nuclear pore complex. Those proteins that form the cytoplasmic fibrils and nuclear cage recognize the molecules that are then allowed to cross the nuclear passage. It should be noticed that molecules coming in and going out of the nucleus do no need to cross any membrane, but just the hydrophilic channel of the nuclear pores.
2. Nucleo-cytoplasm transport
The nuclear pore complex contains a hydrophilic passage ranging from 80 to 90 nm in diameter. When a nuclear pore complex is at rest (no trafficking) the usable space is about 45 to 50 nm in diameter, and it can be expanded when some molecule is being transported. Small molecules (less than 60 kDa) like salts, nucleotides, small molecules and short polypeptides can cross freely through the hydrophilic channel, but larger molecules with physiological roles are not allowed to go across freely. Even some molecules smaller than 20-30 kDa such as histones, tRNAs and small mRNAs may need the involvement of nucleoporins to cross the nuclear envelope. The selective transport, mediated by nucleoporins, is known as passive facilitated transport. It does not need energy. The nuclear pore complex does not determine the direction of the transport, i.e., getting in or out the nucleus, but molecules travel according to a gradient of the Ran proteins (Figure 3). The energy is spent in generating this Ran gradient.

Trafficking through a nuclear pore complex is high, with more than 1000 crossings per second. This movement of molecules across the nuclear envelope is regulated by the gradient of Ran proteins. Ran are involved in both importing and exporting molecules between the nucleus and the cytoplasm. They generate the Ran-GTP/Ran-GDP gradients for the transport, and the generation of these gradients consumes GTP. Ran molecules can be in three states: Ran-GTP, Ran-GDP and Ran. The state of a Ran molecule depends on several enzymes. In the nucleoplasm, Ran-GTP is more abundant, whereas Ran-GDP is concentrated in the cytoplasm (Figure 3).
Karyopherins are a family of proteins, divided in two subfamilies: importins and exportins, which are responsible for selecting the molecules that can cross the nuclear pore complex. Proteins that need to be imported into the nucleus have a particular amino acid sequence, known as entrance signal peptide, and those that need to be exported to the cytoplasm have an exit signal peptide. These short peptide sequences are not identical for all proteins, but similar. The entrance and exit signal peptides are recognized by importins and exportins, respectively. There are members of importins and exportins with different affinity for the import and exit sequences. Nucleoporins do not interact directly with the transported molecules, but with importins and exportins (Figure 4).
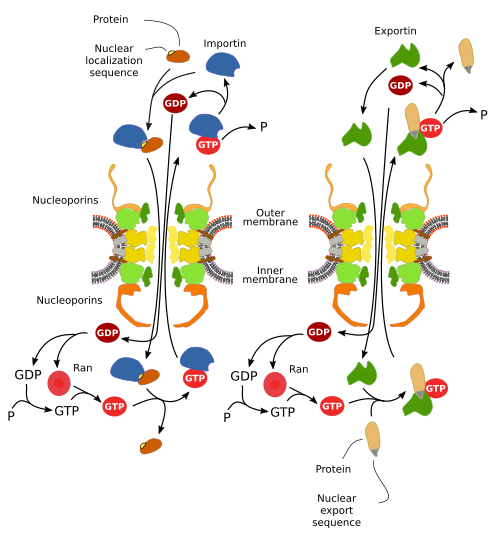
Importins and exportins use the Ran-GTP/Ran-GDP gradients for transporting the cargoes in a specific direction. In this way, importins spontaneously biend their cargoes in the cytosol, but once in the nucleoplasm cargoes are released by Ran-GTP, which is abundant inside the nucleus. On the other hand, exportins need Ran-GTP to join to their cargoes in the nucleoplasm, but once they (exportin-cargo-Ran-GTP) are translocated to the cytoplasm, Ran-GTP is converted into Ran-GDP, which releases the three components of the complex, and then exportin, cargo and Ran-GDP are detached from each other and become free in the cytosol (Figure 4).
Molecules to be specifically transported across the nuclear envelope need to have export or import sequences, but it is not enough. These sequences have to be accessible to exportins and importins. Conformational changes or chemical modifications may hide the sequences, so the molecules remain in the nucleoplasm or cytoplasm until the sequence is properly exposed. This mechanism adds a new step of regulation to nucleus-cytoplasm trafficking.
Besides proteins, other molecules need to cross the nuclear envelope. Different types of RNA are transported by different mechanisms, but there are always proteins involved. mRNA is not exported in a naked form. Once the mRNA transcript is synthesized, it is quickly associated with different nuclear proteins, forming a huge molecular complex known as ribonucleoprotein. Before mRNA is transported, a quality control checks if it has been correctly processed. For instance, if all introns have been removed. In metazoan, the main signal for mRNA exportation is the ending of the mRNA splicing (removing all introns from the primary mRNA transcript). Thus, it is independent of the ribonucleotide sequence. If there are any errors, the mRNA is degraded and it does not reach the cytoplasm. If there are no flaws, the processed mRNA gets associated with 9 proteins that recruit other proteins of the exportin protein family (Nxf1-Nxt1), which facilitate the transport through the hydrophilic channel. This transport does not use the Ran-GTP gradient.
Ehe mRNA associated to importins diffuse through the nucleoplasm until it contacts with the basket of the nuclear pore complex. This interaction is mediated by Nxf1-Nxt1. After this contact, may be a rather long period ending with the release of the mRNA into the nucleoplasm again. However, the mRNA, and associated proteins, may also cross the nuclear pore complex. This crossing does not depend on the ran-GTP gradient. However, it needs ATP as energy source. In the cytoplasm compartment, the large ribonucleoprotein complex containing the mRNA interacts with the cytoplasmic fibrillar proteins of the nuclear pore complex, which leads to the release of Nxf1-Nxt1 and exportins. These proteins go back to the nucleoplasm. There are a small fraction of mRNA molecules that relies on in the CRM1 protein for their exportation. In this case, the process is dependent on the ran-GTP gradient.
Transfer RNA (tRNA) is recognized by a type of exportin, known as exportin-t, which uses the Ran-GTP gradient to get the tRNA out of the nucleus. Small nuclear RNA (snRNA), another type of RNA, is transported by CRM1 protein and Ran-GTP gradient. The exporting mechanism for ribosomal subunits, which are assembled in the nucleolus, is a big challenge for the nuclear pore complexes because they are really large protein-rRNA complexes. The molecular changes happening in the nuclear pore complex allowing the ribosomal subunits to get to the cytoplasm is unknown so far. Finally, it has been shown that some proteins are able to bind directly to nucleoporins, so they do not need neither exportins nor importins.
3. Chromatin interactions
Nuclear pore complexes are involved in additional functions that help in the regulation of the nucleus-cytoplasm communication. In this context, they are thought to participate in DNA repairing, regulation of transcription, and in the processing and quality control of mRNA. At transmission electron microscopy, it is observed that heterochromatin is not distributed near the nuclear pore complexes (Figure 1). Thus, they are regarded as spots of permissive gene expression of many genes. This would be an advantageous position for those genes, since their mRNAs are released close to the gateway. The lack of heterochromatin near the nuclear pore complexes may be a consequence of direct interactions between nucleoporins and chromatin.
Nucleoporins are evolutionary related to COPI and COPII coat proteins, which form the coated vesicles coming from the Golgi apparatus and endoplasmic reticulum, respectively. It makes sense because the nuclear envelope and endoplasmic reticulum membranes are continuous, and Golgi apparatus membranes are formed from vesicles coming from the endoplasmic reticulum.
-
Bibliography ↷
-
Bibliography
Beck M, Lucic V, Forster F, Baumeister W, Medalia O. 2007. Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. Nature 449:611-615.
Cautain B, Hill R, de Pedro N, Link W. 2015. Components and regulation of nuclear transport processes. FEBS journal. 282: 445–462.
Carmody SR, Wente SR. 2009. mRNA nuclear export at a glance. Journal of cell science 122:1933-1937.
Guo T, Fang Y. 2014. Functional organization and dynamics of the cell nucleus. Frontiers in plant biology. vol 5. Artículo 378. doi: 10.3389/fpls.2014.00378.
Kabachinski G, Schwartz TS. 2015. The nuclear pore complex – structure and function at a glance. Journal of cell sciences. 128, 423–429.
Scott DD, Aguilar LC, Kramar M, Oeffinger M. 2019. It’s not the destination, It’s the journey: heterogeneity in mRNA export mechanisms. In The biology of mRNA: structure and function. Eds. Oeffinder M, Zenklusen D. Springer Nature Switzerland AG. DOI: 10.1007/978-3-030-31434-7_2
-
 Nuclear envelope
Nuclear envelope 