When very small tissular structures are going to be visualized, smaller than the light microscope resolution power, such us some organelles, membranes, macromolecular complexes or viruses, we need to make use of electron microscopes. The first electron microscope was made in 1933 by Ruska, and was adapted to study biological samples shortly after. Very small cellular components could be studied, which are commonly called cell ultrastructures. Thus, observing cell ultrastructure means visualizing the cell with an electron microscope. The resolution power of electron microscopes may be as small as 1 nm because they use electron beams, instead of visible light. Electrons have shorter wavelength than visible light and therefore permit several million times magnification. The current electron microscopes are capable of distinguishing atoms in molecules and can reach 50 106 magnifications. In fact, what limits the sharpness of the images is the sample preparation more than the electron microscope itself. Nowadays, there is an intense research for deciphering the 3D organization of molecules by using electron microscopes.
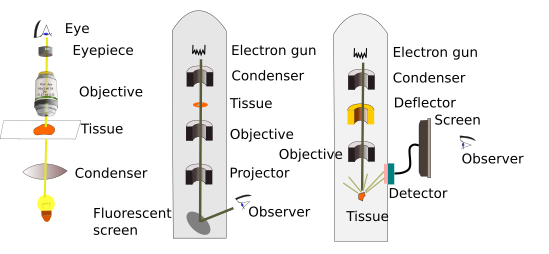
Electron microscopes do not have glass lenses, they use magnets instead, that work as magnetic lenses by concentrating the electron beam emitted by an electron gun. The changes in magnification power are set by modulating the speed of the electrons, which also modifies the wavelength frequency.
Electron microscopes are very large apparatuses because electrons must travel in vacuum. Otherwise, the electrons would hit the particles present in the air. That is why they have large cylinders where the electron beam is formed and manipulated with magnets, and where the sample has to be introduced.
Tow types of electron microscopes are commonly used in histology: transmission electron microscopes and scanning electron microscopes.
1. Transmission electron microscope
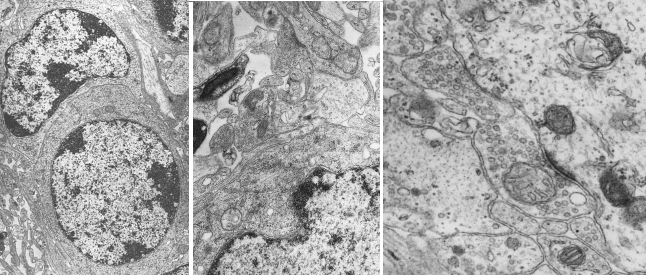
In this type of electron microscopes, a beam of electrons is produced from a tungsten filament that works as a cathode (Figure 1). The electron beam is concentrated by electromagnets and focused on the tissue. Tissue sections have to be very thin, about tens of nanometers, to get sharp images and allow the electrons to cross the whole tissue thickness. That is why they are called ultrathin sections. Previously, sections need to be treated with heavy metals like osmium, lead and uranyl, which have a similar function as dyes in light microscopy, and make cellular structures visible. These metals are mainly deposited in cell membranes and macromolecular complexes. Electrons that go through the tissue are repelled by heavy metals and cannot completely cross the section. Only those electrons that go across the whole section can impact on a fluorescent screen that emits a visible light spark. The image of the section is formed with all the electrons that impact the screen. So, a black and white image is composed (Figure 2): white are non-repelled electrons, black are repelled electrons.
2. Scanning electron microscopy
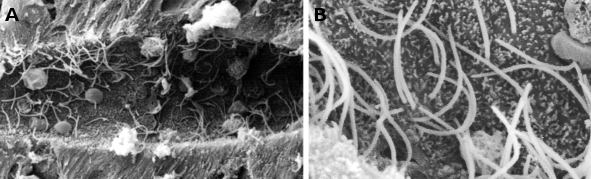
Scanning electron microscopy is used to visualize sample surfaces. This is possible because electrons do not cross the sample, but interact with the surface of the sample. Samples need to have the surface covered with a thin layer of metals. The electron beam scans the surface (that is why the name scanning microscope) and back scattered and secondary electrons are emitted and impact in a detector screen, from which a digital image is formed. The complete image is gotten when the electron beam scans the whole sample. The resolution of these microscopes is an order of magnitude less than that of transmission.
Samples to be observed with scanning electron microscopes are not sections, but portions of tissues. However, surfaces of sections obtained with a vibratome or a freezing microtome can be observed too, but not those coming from paraffin or resin embedding. Although the image is visualized in a screen, and therefore bidimensional, the shadows formed by the scattered electrons form a 3D-like image (Figure 3). It is like a black and white normal photography.
 Light microscopy
Light microscopy