Cells of multicellular organisms are organized in tissues and organs. In animals, this organization largely depends on the ability of cells to adhere to the extracellular matrix or between each other. Cell adhesion relies on transmembrane proteins, known as adhesion proteins, found in the plasma membrane. These proteins made possible the emergence of animals during evolution. In fact, adhesion proteins are very similar when comparing the different animal groups, including marine sponges. Adhesion is not just for anchoring and placing cells to form tridimensional structures, but also for communication between each other. In other words, the type of adhesion and to what a cell is adhered to is a very useful information for the cells.
Cells move through tissues by way of adhesion. Cells don't swim, but crawl. For traveling, cells first need to be attached to some element of the environment, a cell or some molecules of the extracellular matrix, and then drag the nucleus and the rest of the cytoplasm in the direction of moving. During embryo development, cells can move as coordinated groups. In this case, cells travel together by cell-cell adhesion.
Adhesion molecules are found in the plasma membrane. They can diffuse laterally, but get fixed when they get anchored to an extracellular molecule. One adhesion molecule does not make a strong bond, but cell adhesion is performed by many adhesion molecules that altogether make a strong linking, as if they were a molecular Velcro. Some adhesion molecules may interact laterally between each other and form molecular complexes that increase the adhesion strength in some local points of the cell surface. These are structures known as focal adhesions and adhesion junctions. Cells can also regulate the intensity of adhesion and to what molecule they adhere to by way of different mechanisms. For example, cells can change the type and amount of adhesion molecules in the plasma membrane by synthesis, degradation, or hidden them temporarily in internal compartments by endocytosis and exocytosis. Another mechanism to control the strength and specificity of adhesion is by activating or inactivating the adhesion molecules in the plasma membrane.
There are adhesion molecules involved in the attachment of cells to the extracellular matrix and others in linking one cell to another.
1. Cell-extracellular matrix adhesion
Integrins are probably the most important proteins involved in the adhesion of cells to the extracellular matrix, and comprise a large family of transmembrane proteins present in all animals. They are composed of two subunits (alpha and beta) (Figure 1). In mammals, integrins family consists of 18 alpha units and 3 beta units. By combination, they are able to form up to 24 different integrins, which are differentially expressed depending on the tissue and the physiological state of the cell. Integrins have 3 molecular domains. An intracellular domain that interacts with actin filaments of the cytoskeleton (sometimes with intermediate filaments), an extracellular domain that binds collagen, fibronectins and laminins, and an intramembrane domain containing hydrophobic amino acid sequences inserted among the lipid fatty acid chains. The ability of integrins to mechanically connect extracellular matrix and cytoskeleton allows the structural continuity between the internal and external environments of the cell. Furthermore, integrins may change the behavior of the cell according to the molecular composition of the extracellular matrix (they behave like receptors). This is possible because the adhesion state of integrins is transmitted to their intracellular domain by conformational change, triggering molecular interaction cascades in the cytosol, that eventually change gene expression. Cells, in turn, may regulate the strength of adhesion by increasing or decreasing the number of integrins, by synthesizing different integrin subunit subtypes, or by changing the adhesion affinity after modulation of the intracellular domain, which in turn will modify the adhesion ability of the extracellular domain. In general, the adhesion strength of integrins is weaker than that of other adhesion molecules.
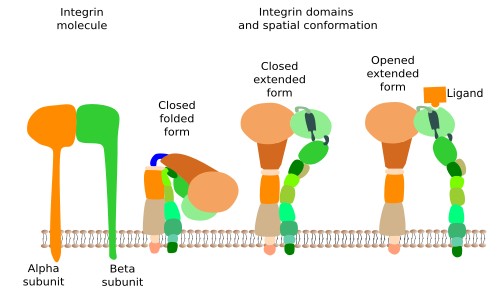
Sometimes, integrins join in groups to form macromolecular complexes known as focal adhesions, and in some cells, like epithelial cells, they form larger complexes called hemidesmosomes. In hemidesmosomes, the cytosolic domains of integrins are connected to intermediate filaments, instead of actin filaments. The strength of the adhesion of a cell to the extracellular matrix depends on the number, the active state and type of integrins that are expressed in the plasma membrane.
2. Cell-cell adhesion.
Some transmembrane molecules establish direct adhesion between cells. There are four types: cadherins, immunoglobulins, selectins and some types of integrins (Figure 2). Cadherins are found in most animal cells and make homotypic contacts, i.e., they recognize and bind other cadherins located in neighboring cells. Cadherins may join laterally between each other at certain points on the cell surface to form groups that result in a greater adhesion strength. There are more than 100 types of cadherins divided in classical and desmosomal cadherins. The name cadherin stands for calcium and adhesion, because they need calcium to make the adhesion contact. Cadherins are a large family of proteins, with some members specifically expressed in particular tissues. For example, N-cadherins are found in the nervous tissue, and E-cadherin in the epithelial tissue. This is why they play an important role during segregation of cell populations in tissues during development, but also in adults. Cadherins are particularly relevant during embryonic development. They are also found as structural parts of desmosomes (macula adherens) and adherent junctions (zonula adherens).
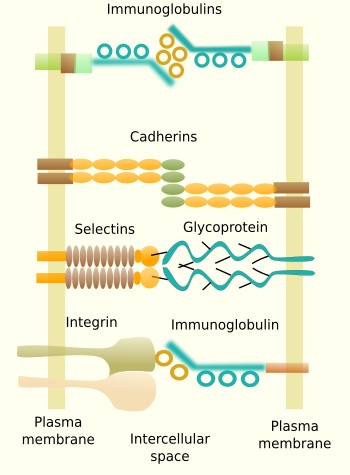
Some adhesion proteins belong to the immunoglobulin family and make homotypic contacts with other immunoglobulins located in neighboring cells, although they can also make heterotypic contacts. They are a large and diverse family of proteins with selective tissue distribution. For example, N-CAM (neuronal cell adhesion molecule) is expressed in the nervous system. The binding strength of immunoglobulin proteins is weaker than cadherins, and it is thought to be suitable to fine tune the segregation of cells into groups inside tissues. Selectins are another type of adhesion molecules involved in cell-cell adhesion by heterotypic contacts. They bind carbohydrates (sialic acid and fucose) located at the surface of adjoining cells. For example, selectins are needed during the exit of leukocytes from blood vessels toward the extracellular matrix of surrounding tissues. Some integrins do not participate in cell-extracellular matrix adhesion, but are involved in cell-cell adhesion. For example, they can make heterotypic contacts with certain types of immunoglobulins of neighboring cells.
Ocludins and claudins are cell-cell adhesion molecules mainly found in tight junctions, which are adhesion complexes present in epithelia and other tissues.
 Transport
Transport