1. Permeability
2. Fluidity
3. Heterogeneity
- Inner
- Outer
- Bending, thickness
Cell membrane functions are determined by their molecular composition. In this page, we are dealing with three membrane features: semipermeability, fluidity and lateral heterogeneity.
1. Permeability
Membrane semipermeability is a consequence of their inner hydrophobic environment generated by the lipid fatty acid chains. This hydrophobic space is difficult to be crossed by molecules having net electric charge. Thus, by preventing free diffusion of some molecules, membranes can form compartments that keep distinct internal and external environments, as well as an intracellular milieu different from that of the extracellular space. However, permeability is selective, that is, not all molecules have the same difficulty to cross the membrane. Polarity and size are the more important molecular features that influence the ability to cross membranes. Small molecules without electric charges, such as CO2, N2, O2, and molecules with high solubility in fat, such as ethanol, can cross membranes almost freely by passive diffusion (Figure 1). Permeability is lower for molecules that have electric charges when the number of positive charges equals negative charges, such as water and glycerol. It might be thought that water can cross membranes freely, but there are some restrictions, and that is why some membranes contain aquaporins, a type of transmembrane protein with a channel that allow the water to cross freely. The ability of large uncharged molecules, such as glucose, to cross membranes is lower. Membranes are highly impermeable to ions and net charged molecules. Some values for the permeability coefficient by passive diffusion are: O2: 2.3 cm/s, CO2: 0,35 cm/s, H2O: 0,0034 cm/s, glycerol: 10 -6 cm/s, sodium and potassium: 10 -14 cm/s.

The unequal distribution of ions and molecules between both sides of a membrane leads to the electrochemical gradients. The difference between the inner and the outer concentration of electric charges is known as membrane potential. This gradient is used for many cell functions, such as ATP synthesis and transmission of information along the nerves. Semipermeability is also responsible for osmotic processes, which are water movements across membranes from a less concentrated solution in one side to a more concentrated solution in the other side, in order to equal concentrations. In this way, plant cells are able to increase in size. Molecules that do not cross membranes freely are useful for cells because they can form gradients that may work as information signals or as tools for other processes. Cells have transmembrane proteins that can break gradients by selectively allowing some ions or molecules to cross the membrane. For example, muscle contraction is triggered by the opening of channels that reduce an existing ionic gradient. Transmembrane proteins can also generate gradients by transporting charged molecules and ions across cell membranes against concentration gradient, avoiding the inner membrane hydrophobic environment.
Semipermeability is influenced by the lipid composition. More fluid membranes (see below) are more permeable. For example, cholesterol content is important in the plasma membrane. An increase in the cholesterol content decreases fluidity and increase hydrophobicity, both features making the membrane more impermeable. Thus, the increase of cholesterol over 30 % (which is a high value) makes myelin membranes very suitable for insulating the axons and optimizing the propagation of the action potential along the axon.
2. Fluidity
Fluidity is one of the membrane features. It is related to the ability of molecules to move within membranes. Higher fluidity means that movements are more frequent. Cell membranes are actually a sheet of fat, where molecules are in a semi liquid viscous state. Thus, it can be guessed that molecules can move by diffusion. A glycerophospholipid located in the external hemilayer of the plasma membrane may have two type of movements: lateral, i.e., in the same hemilayer, and flip-flop, i.e., jumping from one hemilayer to the other (Figure 2). In artificial membranes, both types of movements have been observed, being the lateral movements much more frequent than flip-flop. By lateral diffusion, lipids can travel 30 µm in 20 seconds; they can travel the whole circumference of a medium size cell in a minute. On the other hand, flip-flop movements are really infrequent because the hydrophilic head of the lipid molecule must cross the internal hydrophobic layer of fatty acid chains, and this is thermodynamically difficult. For one lipid molecule, the probability of a flip-flop shift is about one time per month. However, cholesterol behaves different and can do flip-flop quite easily.
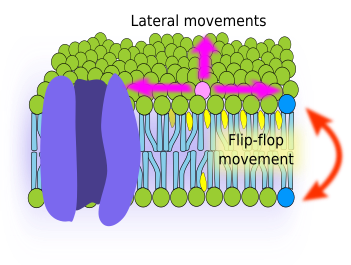
Fluidity may change depending on the chemical composition of the membrane. Generally, shorter fatty acid and higher amount of unsaturated bonds between carbons of fatty acids increase membrane fluidity. The amount of cholesterol also influences the membrane fluidity, but the net effect depends on temperature and type of lipids in the membrane. Cholesterol has two effects: inhibits the transition to a solid gel state (less fluidity), but also decreases the flexibility of the unsaturated fatty acid chains. In general, it can be said that an increase in cholesterol concentration decreases membrane fluidity, although at low temperatures the effect is the opposite. Internal membranes of the cell, like those of the endoplasmic reticulum, contain very little cholesterol, and therefore they are more fluid. In addition, cholesterol provides membranes with another property known as hydrophobicity, which makes membranes more impermeable.
Different molecular composition between the two hemilayers of a membrane, known as membrane asymmetry, may lead to a distinct fluidity in each hemilayer. Depending on the molecular composition, lipids may be in two physical phases: liquid-ordered (less fluidity) and liquid-disordered (more fluidity). The outer hemilayer of the plasma membrane is supposed to be more often in the liquid-ordered phase, whereas the inner hemilayer is prone to be in the liquid-disordered phase.
Cells can modify the membrane fluidity by changing the chemical composition. For example, bacteria adjust the saturation and length of fatty acid chains so that membrane fluidity is adapted to the environmental conditions. Changes in the concentration of some types of glycerophospholipids, such as phosphatidylethanolamine, may also modulate fluidity. Thus, some insects cannot synthesize sterols, like cholesterol, but they can modulate the fluidity of their cell membranes by modifying the concentration of phosphatidylethanolamine.
The inner membrane of span mitochondria needs to work as a strong, impermeable barrier for generating and maintaining a proton gradient. It would be done by an increase in the cholesterol content. In this way, the hydrophobicity is higher. However, cholesterol decreases fluidity, feature that appears to be very important for the function of the proteins in this membrane. Mitochondria solve the problem with span cardiolipin, an unsaturated phospholipid that increases hydrophobicity, but does not reduce much the membrane fluidity.
3. Heterogeneity
BBecause of fluidity, it can be thought that molecules are randomly distributed and, therefore, membranes are homogeneous regarding the molecular composition, i.e., they show the same molecular content and proportion independently of the membrane region. This is not true. There are restrictions to the lateral diffusion of molecules that causes membrane heterogeneity, which means that there are regions of a membrane with different molecular composition. In non polarized cells, and at scales larger than 200 nm, plasma membrane looks like homogeneous. However, we can find large scale domains, such as synapses of immune cells and cell junctions. At scales below 200 nm, membranes show a patchy organization, so that they are heterogeneous. The microdomains of membranes are thought to be around 60 nm to 100 nm in size. Lipids and proteins show lateral movements mostly restricted to 60-100 nm areas during a few milliseconds, and then jump to another adjoining area where they remain for another short time. This behavior is called saltatory diffusion. It is also known that some molecules practically remain in the same position for long times, whereas other molecules are free to diffuse laterally.
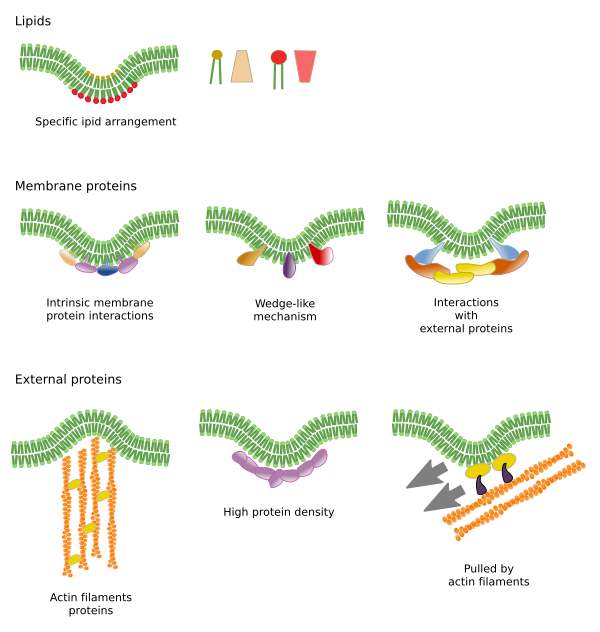
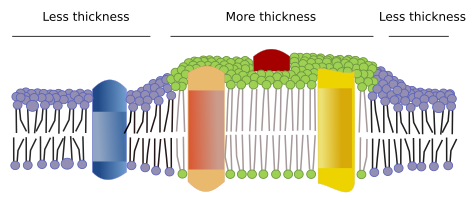
Restrictions to lateral movements of molecules may be caused by several mechanisms: interactions with the cytoskeleton or extracellular matrix, interactions of membrane molecules between each other, different densities of local membrane areas (changes in fluidity), amount of electrical charges, membrane curvature, and different thickness of membrane domains (Figure 3).
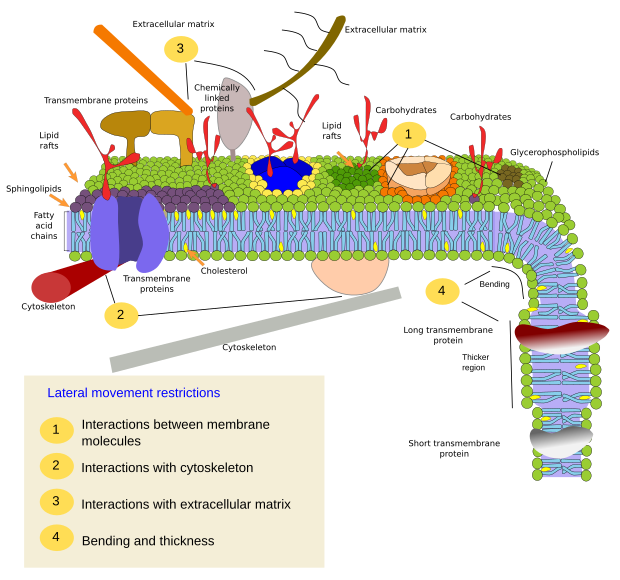
Inner interactions
Interactions of membrane molecules between each other constrain lateral movement (Figure 4). Both, proteins and lipids movements are influenced by these interactions, leading to the formation of microdomains with differential molecular composition. Depending on molecular proportions, microdomains may have different density: solid, liquid ordered and liquid disordered. The most common state is liquid disordered, which is the most fluid.
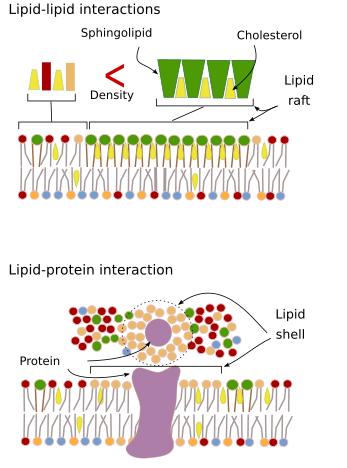
Sphingolipids and cholesterol may become associated spontaneously between each other, reducing their motility and increasing the molecular density when compared to neighbor areas. A small, distinct group of molecules is formed, like a raft in a sea of lipids. Actually, these molecular associations are known as lipid rafts, and are thought to be very abundant in the plasma membrane, forming a mosaic of microdomains. Lipid rafts are very small, between 10 nm and 200 nm, and show a highly dynamic behavior, so that they can move, grow, shrink, appear and disappear. Some experiments suggest that certain types of proteins "feel" more comfortable inside lipid rafts. These proteins spend more time inside than outside the lipid rafts, thus they travel for some time within these microdomains. It leads to a segregation of molecules along the membrane, and increases the probability of different molecules to be close to each other more time than just by chance (diffusion), increasing in this way the probability of certain molecular reactions. It is suggested that a high concentration of certain types of lipids in the lipid rafts forms a distinct chemical environment that makes easier some chemical reactions or molecular interactions. For example, the infection of lymphocytes by the AIDS virus is favored by these microdomains, where the virus is easily anchored. Lipid rafts have been proposed to be only present in the outer hemilayer of the plasma membrane because it is in this side where sphingolipids are abundant. Membrane domains have also been suggested in the membranes of some organelles, and it is thought that some of their functions rely on these membrane domains.
In the inner hemilayer of the plasma membrane, microdomains are formed by electrostatic interactions between basic cytosolic domains, or divalent cation domains, of proteins and the negative polar heads of lipids. Phosphaotidilinositol is involved in the formation of microdomains associated to proteins. By adding or removing phosphate groups in the head of this lipid type, the microdomains can be modulated. Another less known example is the association between phosphatidylinositol bisphosphate and cholesterol that forms microdomains in the inner hemilayer of the plasma membrane. These microdomains facing the cytosol may influence intracellular protein scaffolds.
Traditionally, it has been thought that there are no interactions between both hemilayers of membranes, so that they distribute their microdomains independently. However, evidences suggest that there are interactions between each other. For instance, transmembrane proteins simultaneously affect both hemilayers since they cross the entire membrane. Other way of synchronizing both hemilayers may be mediated by the long fatty acid chains, like some sphingolipis that can be 24 carbons in length (the normal length is about 18). These long chains may be inserted among the fatty acid chains of the lipids of the other hemilayer and influence the lipid distribution. Furthermore, lipid domains with long fatty acid chains are thought to be counterbalanced by short fatty acid chain lipid microdomains in the other hemilayer, keeping constant the thickness of the membrane.
Membrane proteins, both integral and associated, may also interact between each other and assemble into macromolecular scaffolds to facilitate transmission of information, cell-cell recognition, initiate some enzymatic activities, and cellular movement. There are also multimeric proteins, active only when all the subunits are hold together. For example, the insulin receptor is made up of four subunits.
Interactions with outer elements
Integral membrane proteins may also have lateral movements, but they are more restricted than lipids, mostly due to interactions with the extracellular matrix and cytoskeleton through their extracellular and intracellular domains, respectively (Figure 5). These interactions may keep proteins in small areas of the membrane for a longer time than just by diffusion. Cytoskeleton may also form fences just below the plasma membrane that keep proteins restricted to small areas.
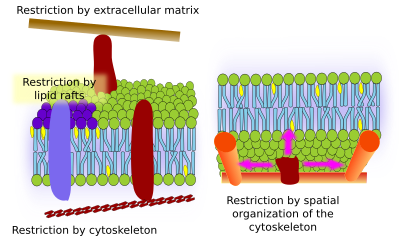
There is a scaffold of myosin and actin (cytoskeleton proteins) beneath the plasma membrane that interacts with membrane proteins and restrain their movements. Although temporally, these retained proteins may act as obstacles for the diffusion of other membrane molecules. In this way, molecular interactions may lead to immobilize large molecule aggregates or to move them through the membrane, propelled by the cytoskeleton. For instance, cilia contain particular sets of proteins thanks to these type of interactions with the cytoskeleton. When the cytoskeleton is disorganized, the membrane become more homogeneous.
There are other mechanisms to confine proteins in specific regions of the membrane. For instance, the epithelial cells of the digestive tube concentrate some transporters and enzymes in the apical domain (the free surface), whereas others are confined to the baso-lateral domain. It means they are polarized cells. The segregation of proteins between the two domains is caused by tight junctions, cell-cell junctions that form a belt-like structure around the cell that can not be crossed by proteins. This polarization is essential for the intake nutritive substances after digestion.
The plasma membrane molecules of the outer hemilayer interact with molecules of the extracellular matrix, such as collagen, proteoglycans, hyaluronic acid, and many others.
Bending and thicknes
Cending a membrane is another way to form microdomains. The initial bending may be the beginning to generate a vesicle, a cellular extension, the growing of an organelle, or just to make a fold as a barrier for lateral diffusion.
Che molecular machinery for bending a membrane has to be recruited to a previous microdomain. Some lipids and electric charges concentrated in son membrane sites recruit the bending proteins. Phosphoinositides (PI) participate in the this recruitment, particularly PIP2 and PIP3. They can change the electric charge of their polar heads quite easily by local chemical modifications. Phosphatidilserine is also involved in the beginning of membrane bending when it is transported by flipases from one hemilayer to the other hemilayer. Both, phosphoinositides and phosphatidylserine can be retained in the bending site by the recruited proteins.
Lipid microdomains recruit proteins that effectively bend the membrane (Figure 6). They are specialized proteins, such as BAR-domain proteins (Bin/amphyphysin/Rsv161). Bending may be induced by two mechanisms: assembling of a scaffold of proteins that pull or push the membrane, or by inserting amino acid sequences among the lipids as a wedge. For example, caveolins cause membrane curvature to form caveloae, tetraspanin force membranes to form tubules, ESCRT complex helps with vesicle formation within endosomes to form multivesicular bodies. Actin is another strong membrane bending agent by actin filament polymerization that pushes the plasma membrane outward, leading to cell expansions. Many proteins that can curve the plasma membrane also activate actin polymerization.
There are other microdomains in membranes formed by transmembrane proteins (Figure 7). These regions show different membrane thickness because they contain proteins with longer hydrophobic amino acid sequences that get surrounded by lipids with longer fatty acid chains. The proteins and lipids "feel" more comfortable when they are together, since they fit properly their hydrophobic parts. They form membrane domains that exclude other molecules, either proteins or lipids, with shorter hydrophobic regions.
-
Bibliography ↷
-
Bibliography
Honigmann A, Pralle A. 2016. Compartmentalization of the cell membrane. Journal of mollecular biology. 428: 4739-4748.
Jacobson K, Liu P, Lagerholm BC. 2019. The lateral organization and mobility of plasma membrane components. Cell. 177: 806-819. DOI: 10.1016/j.cell.2019.04.018.
Jarsch IK, Daste F, Gallop JL. 2014. Membrane curvature in cell biology: an integration of molecular mechanisms. Journal of cell biology. 214: 275-387
Nicolson GL. 2014. The Fluid—Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochimica et biophysica acta. 1838: 1451-1466.
Subczynski WK, Pasenkiewicz-Gierula M, Widomska J, Mainali L, Raguz M. 2017. High cholesterol/low cholesterol: effects in biological membranes: a review Cell biochemstry and biohysic. https://doi.org/10.1007/s12013-017-0792-7
-
 Carbohydrates
Carbohydrates