1. Fibronectins
2. Laminins
3. Matricellular
- Tenascin
- Osteopontin
- Fibulin
4. Metaloproteinases
There are three adhesion mechanisms to maintain the structural integrity of tissues: cell-cell adhesion, cell-extracellular matrix adhesion, and linkages between molecules of the extracellular matrix. The extracellular matrix is a cohesive network of molecules linked to each other. Glycoproteins greatly contribute to make the extracellular matrix a cohesive network of molecules. They are intermediaries that link structural molecules between each other, and also link structural molecules and cells. In each glycoprotein molecule, there are several domains binding different molecules that altogether forms cross-linked molecular networks. Although cohesion is a main function, glycoproteins perform many other functions.
1. Fibronectin
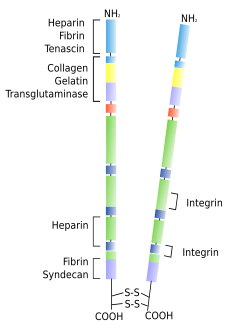
Fibronectins are glycoproteins consisting of two polypeptides joined by disulfide bridges (Figure 1). They are major players in making the extracellular matrix cohesive. Fibronectins have several molecular domains that recognize and bind glycosaminoglycans, proteoglycans, fibrin, heparin and some transmembrane proteins such as integrins. Therefore, fibronectins are intermediaries that make connections between different extracellular matrix molecules, but also between extracellular matrix and cells. Fibronectins are found in almost every tissue. They can be found as insoluble fibers in connective tissues, and soluble molecules in body fluids such as blood. Besides maintaining extracellular matrix cohesion, fibronectins have many other functions. For example, during embryonic development, cells move from one side to another through adhesion paths made of fibronectin. They are also important during tissue remodeling.
2. Laminin
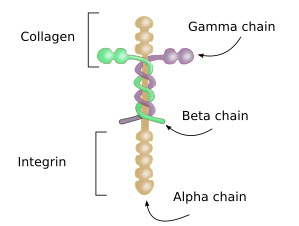
Laminins are a major component of the basal lamina, the extracellular matrix layer that separates epithelia and muscle cells from connective tissues. It consists of three highly glycosylated polypeptides: alpha, beta and gamma, joined together by disulfide bridges (Figure 2). There are 5 types of alpha chains, 3 of beta chains, and 3 of gamma chains, that are combined to form different types of laminins, although not all combinations may be possible because only 16 laminin subtypes have been found in humans. Laminins are synthesized by epithelial cells, muscle cells, neurons, and bone marrow cells. Epithelial and muscle cells release laminins to the basal lamina. Besides the structural function in the extracelular matrix, laminins also influence cell behavior and differentiation through interactions with integrins. That is why mutation of laminins usually leads to pathological processes. During embryonary development, laminins are the first type of glycoprotein to be released to the extracellular matrix.
3. Matricellular glycoproteins
Around the nineties of the XX century, it was observed that some glycoproteins of the extracellular matrix decrease the adhesion strength of cells. These proteins have domains for binding collagen, fibronectin and some cell receptors, but their main function is not to keep structural integrity of the extracellular matrix. They are released into the extracellular matrix during particular periods of time and in specific places by many types of cells. Therefore, they are not constitutive but temporary proteins in the extracellular matrix.
One major function of matricellular proteins is modifying the cell activity and facilitating the extracellular matrix remodeling. These processes take place during the embryonary period, in normal tissue development, and during tissue repairing after pathological damage. Some of these proteins influence the cell activity even before they are released to the extracellular matrix, i.e., in the intracellular compartments. Tenascin, trompospondin, osteopontin, fibulin, and SPARC protein are matricellular proteins.
Tenascin
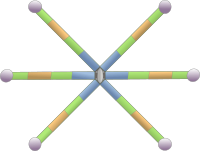
Tenascins form a family of large molecular size glycoproteins located in the extracellular matrix of animals. The molecular structure shows a modular hexameric organization (Figure 3). Several isoforms of tenascin can be obtained by alternative splicing of the messenger RNA. Tenascin-C was the first tenascin isoform discovered. It is released into the extracellular matrix of tendons, bones and cartilage during the embryonary development. However, it can also be found in other tissues. Although it is very scarce in adult tissues, tenascin-C is over expressed as a consequence of tissue damages like heart attack. Tenascin-R is abundant in the nervous system, both during development and in adults. Tenascin-X is present in the connective tissue and can be abundant in muscles under heavy activity, like in professional athletes. Teanscin-Y and -W have also been described. Tenascin-Y is homologous to avian tenascin-X. Like other glycoproteins, tenascins change the cohesive state of the extracellular matrix by binding integrin, fibronectin, collagen and proteoglycans. In animals, each type of tenascins are expressed in particular locations of the organism, that may change during development. The expression of tenascins is induced in tissues being repaired, or during tumor and pathological processes.
Osteopontin
Osteopontin is found in bones, involved in mineralization and remodeling, and in kidneys. A lower amount is also present in cartilage, where it recognizes proteoglycans.
Fibulin
Fibulins form a group of 7 glycoproteins associated to the basal lamina, elastic fibers and other components of the extracellular matrix. Each of the 7 members shows a differential expression both in different tissues and during development. Fibulin 5 is important for elastic fibers because it can bind tropoelastin. Besides the influence in the extracellular matrix network, fibulins modulate the cell behavior, so that these molecules may work as matricellular and structural proteins.
4. Metalloproteinases. Matrix remodeling.
The extracellular matrix of animals is in constant turnover by a process of degradation and synthesis of molecules, which are under the control of the cell. Degradation is carried out by enzymes, such as metalloproteinases. These enzymes are either exocytosed and then associate with the external layer of plasma membrane as free molecules, or they can be found inserted in the plasma membrane. In both cases, the catalytic domain faces the extracellular matrix. Metalloproteinases are synthesized as inactive forms, known as prometalloproteinases, and are activated by proteolytic cleavage, which is carried out by enzymes associated to the plasma membrane. In mammals, there are more than 29 types of metalloproteinases, which degrade different types of extracellular matrix molecules. Tthere are collagenases, gelatinases, stromalysines, matrilysins, transmembrane metalloproteinases, membrane associated enzymes, and a group with a diversity of members (Figure 4). A type of metalloproteinase can degrade several types of molecules, but it is mostly active on one type. Metalloproteinases are also able to degrade membrane receptors, adhesion proteins, cytokines, and many other molecules.

Besides maintaining the extracellular matrix homeostasis, metalloproteinases are key players in the extracellular matrix remodeling after certain signals, such as hormones, during pathological processes like inflammation, during repairing wounded tissues, in tumor metastasis, and during embryonic development. Another role of metalloproteinases is to release molecules that are retained by the extracellular matrix, which become signals for neighbor cells. Metalloproteins are produced by fibroblasts, but also by epithelia, chondrocytes, osteoblasts, leukocytes, as well as by cancer cells.
-
Bibliografía ↷
-
Bibliografía
Berg G, Barchuk M, Miksztowicz V. 2019. Behavior of metalloproteinases in adipose tissue, liver and arterial wall: an update of extracellular matrix remodeling. Cells 8: 158. doi:10.3390/cells8020158.
Bosman FT, Stamenkovic I. 2003. Functional structure and composition of the extracellular matrix. Journal of pathology. 200:423-428.
Halper J, Kjaer M. 2014. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. In: Halper J. (eds) Progress in heritable soft connective tissue diseases. Advances in experimental medicine and biology, vol 802. Springer, Dordrecht
Hynes RO. 1999. Cell adhesion: old and new questions. Trends in cell biology . 9:M33-M37.
Luo BH, et al. 2007. Structural basis of integrin regulation and signaling. Annual review of immunology. 25:619-647.
Midwood KS, Chiquet M, Tucker RP, Orend G. 2016. Tenascin-C at a glance. 129: 4321-4327.
Mouw JK, Ou G, Weaver VM. 2014. Extracellular matrix assembly: a multiscale deconstruction. Nature reviews. Molecular cell biology. 15:771-785.
Murphy-Ullrich JE, Sage EH. 2014. Revisiting the matricellular concept. Matrix biology. 37:1-14.
Pankov R, Yamada KM. 2002. Fibronectin at a glance. Journal of cell science. 115:3861-3863.
-
 Carbohydrates
Carbohydrates 