In the late XIX century, a barrier limiting the nucleus was suggested, and was later confirmed by electron microscopy (Figure 1). The nuclear envelope is composed of two membranes and performs a number of functions: a) it is a physical barrier that separates the nucleoplasm (chromatin and the rest of the molecular content of the nucleus) from the cytoplasm; b) it regulates the communication between nucleoplasm and cytoplasm, that is, the movement of molecules between both compartments; c) it is in charge of the nuclear morphology and position of the nucleus in the cell; d) it contributes to the inner organization of the nucleus providing the anchoring points where chromatin is attached.

1. Components
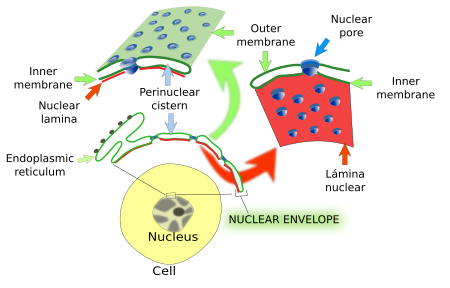
The nuclear envelope consists of an outer membrane, an inner membrane, and an intermembrane space (25-40 nm) between them. All together, they form the so-called perinuclear cisternae (Figure 2). The outer membrane is continuous with the membrane of the endoplasmic reticulum and may contain attached ribosomes. The lumen of the endoplasmic reticulum and the lumen of perinuclear cisternae are also continuous. Thus, the nuclear envelope may work as a calcium-storing compartment, along with the endoplasmic reticulum. That is why the nuclear envelope is regarded as a domain of the endoplasmic reticulum. The inner membrane has a distinct molecular composition. For example, there are transmembrane proteins linked to chromatin and to the nuclear lamina, another component of the nuclear envelope (see below). The inner and outer membranes are continuous at the insertion place of the nuclear pore complexes. How is it possible a different molecular composition between the outer and inner membranes? There is a selective retaining molecular mechanism in the inner membrane. Transmembrane proteins are synthesized in the outer membrane of the nuclear envelope or in the rough endoplasmic reticulum, and arrive to the inner membrane by lateral diffusion (thanks to the membrane continuity), but those that become linked to chromatin or to the nuclear lamina are retained in the inner membrane of the nuclear envelope.
IIn animal cells, the nuclear lamina is a fibrous molecular scaffold found between the inner membrane and chromatin. In mammals, nuclear lamina is around 20 - 25 nm thick. The main components of nuclear lamina are proteins known as laminas, with two isoforms: type A (A and C laminas, which result from the alternative splicing of the same mRNA, i.e., the same gen) and type B (B1 and B2/B3 laminas). B laminas are essential for the cell because animals lacking B laminas are not viable. All laminas are members of the type V intermediate filament family. They are organized as a net lining the inner surface of the inner membrane of the nuclear envelope, linking the inner membrane and the chromatin. The attachment between the inner membrane and the nuclear lamina is mediated by at least 20 different types of proteins inserted in the inner membrane. Laminas are evolutionary old proteins, and it has been suggested that they gave rise to the other intermediate filaments of the cell.
The nuclear lamina performs multiple functions:
- It contributes to keep the organization of the nuclear envelope, and therefore the shape and size of the nucleus. The nuclear morphology changes when the expression of proteins of the nuclear lamina is altered, which can be observed during embryo development, cell differentiation and some cell pathologies.
- The nuclear lamina is a place for anchoring the nucleus to the cytoskeleton, allowing to place the nucleus in a precise location within the cell, as well as moving the nucleus from one place to another. This connection nuclear lamina-cytoskeleton is mediated by proteins inserted in the membranes of the nuclear envelope.
- The spatial distribution of nuclear pore complexes in the nuclear envelope is also influenced by the nuclear lamina.
- The nuclear lamina performs several functions affecting the chromatin. For example, it provides physical support for chromatin, which affects gene expression. The chromatin region anchored to nuclear lamina is not usually transcribed. These chromatin regions are different depending on the cell type and differentiation state of the cell, so that the nuclear lamina-chromatin interactions may be regulatory elements of the gene expression. About 40 % of the human chromatin is heterochromatin associated to the nuclear lamina. These interactions involved chromatin proteins such as H2, H3, H4 histones, HP1 (heterochromatin protein), and BAF, and proteins of the nuclear lamina, such as LAP2, emerin, and MAN1. Altogether, it indicates that the nuclear lamina is a main actor in the regionalization of the nucleoplasm.
- During mitosis, the nuclear envelope should be disassembled and assembled again. This process is mediated by enzymatic action (phosphorylation) on the laminas that causes breakdown of the nuclear lamina, so that microtubules can contact with chromosomes.
Pathological alterations of laminas, mainly type A laminas, result in the so-called laminopathies. Laminopathies may be degenerative diseases affecting specific tissues: muscle distrophy, lipodistrophy, peripheral neuropathies, cardiomyopathies, dermopathies, or to many tissues at the same time as in progeria. The genes affected may alter the nuclear integrity, as well as the chromatin organization and gene expression.
Nuclear pores are inserted in the nuclear envelope. They are in charge of the trafficking between the cytoplasm and nucleoplasm (see next page).
2. Function
Since it involves quite an amount of resources, why do eukaryotic cells need to separate the DNA from cytoplasm? Some of the reasons are the following:
a) DNA stability. Confining the genome inside a compartment helps to maintain the stability of the DNA, which is higher than in prokaryotes; it should keep in mind that there is a huge amount of DNA.
b) Gene regulation. Separation of the genome from the cytoplasm rises the gene regulation at a level that prokaryotes will never reach. For example, it prevents or allows the access of transcription factors to DNA. Transcription factors are proteins synthesized in the cytoplasm that regulate gene expression. They must cross the nuclear envelope to work on the DNA. The molecular mechanisms that activate a transcription factor to enter the nucleus is usually the result of an array of molecular interactions that may start with the activation of a receptor located in the plasma membrane. If some step of this chain is stopped, the gene will not be expressed.
c) Eukaryotic genes contain exons and introns, meaning that a maturation process (cutting and splicing) of the primary mRNA is needed. It is dangerous to translate an unprocessed mRNA because it may produce malformed proteins, which can even cause pathologies. This mRNA processing takes place in the nucleoplasm, and only mature mRNA is allowed to cross the nuclear envelope.
d) Transcription and translation take place in separate compartments (nucleoplasm and cytosol, respectively). The nuclear envelope provides an additional step for regulating the flux of information from DNA to proteins. In this way, the transcription of a gene into mRNA does not mean immediate translation. For example, preventing a particular mRNA to cross the nuclear envelope means that the cell will not get the protein right away.
e) The nuclear envelope is a major regulator of the organization of chromatin into chromatin territories, and contribute to its tridimensional arrangement in the nucleoplasm, therefore influencing the overall gene expression. The contacts of chromatin with the nuclear lamina are the base for this role in the chromatin organization.
f) Position, shape and size of the nucleus (see below).
3. During mitosis
In most eukaryotic cells, the nuclear envelope breaks into little vesicles during the mitotic prophase. It is known as open mitosis because cytosolic microtubules can gain access and make contact with chromosomes. Once chromosomes are segregated, two new nuclear envelopes are assembled again during telophase from the membranes of the endoplasmic reticulum to form the nuclei of the two daughter cells (Figure 3). In yeasts and other eukaryotes, however, the integrity of the nuclear envelope is maintained, and all the molecular machinery for chromosome segregation has to be imported into the nucleus. The mitotic spindle is intranuclear, and the two new nuclei are formed by strangulation. It is known as closed mitosis.

4. Nuclear position
The nucleus can be found in different places in the cytoplasm depending on the cell type, cell activity, and differentiation stage. Sometimes the nucleus is passively displaced by other components of the cytoplasm, like large lipid droplets of adipocytes and myofibrils in skeletal muscle cells, both cell types having the nucleus close to the plasma membrane. In general, the nucleus is actively placed in a specific region of the cytoplasm by the interaction of the cytoskeleton with the nuclear envelope, largely by actin filaments and microtubules, but intermediate filaments can participate as well. In animal cells, the nuclear envelope may be connected with the centrosome, which drags the nucleus along the microtubules. Microtubules can also contact directly with the nuclear envelope. The nuclear movement is the result of the activity of the motor proteins associated to the cytoskeleton, although short distance movements may be elicited by polymerization and depolymerization of cytoskeletal filaments. Proteins in the membranes of the nuclear envelope are intermediaries between the cytoskeleton and the nuclear lamina. Nuclear movements ranging from 0.1 and 1 µm/min have been recorded. The faster movements, 10 µm/min, are those of the pronuclei in the oocytes after gamete fusion during fertilization.
There are several protein complexes anchoring the nuclear lamina to the cytoskeleton. They include the KASH transmembrane proteins (nesprins in mammals) in the outer membrane, and the SUN proteins in the inner membrane of the nuclear envelope. There may be more proteins involved in these cytoskeleton-nuclear envelope bridges (Figure 4).

In mammals, 5 genes code for KASH proteins, some of them showing mRNA alternative splicing that can generate quite long protein isoforms exceeding 800 kDa. KASH proteins have very long molecular processes that extend into the cytosol and contact the cytoskeleton. SUN proteins link to KASH proteins in the perinuclear space (between the two membranes of the nuclear envelope), and to laminas (proteins of the nuclear lamina) by their nucleoplasmic molecular domain.
-
Bibliograpy ↷
-
Bibliography
Gundersen GG, Worman HJ. 2013. Nuclear positioning. Cell. 152: 1376-1389.
Guo T, Fang Y. 2014. Functional organization and dynamics of the cell nucleus. Frontiers in plant biology. vol 5. Artículo 378. doi: 10.3389/fpls.2014.00378
Rowat AC, Lammerding J, Herrmann H, Aebi U. 2008. Towards and integrated understanding of the structure and mechanics of the cell nucleus. BioEssays 30: 226-236.
Starr DA, Fridolfsson HN. 2010. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annual review of cell developmental biology. 26: 421-444.
Wanke C, Kutay U. 2013. Enclosing chromatin: reassembly of the nucleus after open mitosis. Cell 152: 1222-1225.
Wilhelmsen K, Ketema M, Truong H, Sonnenberg A. 2006. KASH-domain proteins in nuclear migration, anchorage and other processes. Journal of cell science 119: 5021-5029.
-
 Nucleus
Nucleus