Light, or optical, microscopes are essential for histological studies because they allow us to visualize cells and morphological features of tissues. Light microscopes relies on glass lenses and visible light to magnify tissue samples. It was invented in XVII century, and has been improved over the years, resulting in the powerful modern light microscopes. The most remarkable improvement has been getting better glass lenses to obtain sharper and non-distorted images, as well as adding devices to explore new ways of visualizing tissular features.
The resolution power of light microscope is 0.2 µm. It is the shortest distance between two points where they can be distinguished from each other. This limit is a consequence of visible light wavelength.
Light microscopes contain two main lenses: objectives and eyepieces (ocular). Objective gathers the light that went through the tissue, whereas the eyepieces project the tissue image on the eye. The total magnification is the result of multiplying the objective magnification by the eyepiece magnification. For example, if we have a 40x objective (magnifies 40 times) and a 1x eyepiece (magnifies 10 times), the total magnification is 400 times. More advanced microscopes can get 1000 to 1500 magnifications (100x objective and 10x or 15x eyepiece). Some light microscopes may contain additional internal lenses between the objective and the eyepiece that can change the total magnification. Magnification and resolution power must not be confused, because not matter how we can magnify an image, including digital methods, the resolution cannot be increased.
1. Light microscope parts
A common light microscope is made up of the following components:
Eyepieces. They are the lenses that form the final image projected on our retina (Figure 1). All current light microscopes bear two eyepieces. Therefore, they are called binoculars. The first microscopes were monocular, that is, they only had one ocular. Nowadays, both eyepieces and objectives are composed of several lenses. At least, one of the eyepieces can be regulated to get closer or away from the objective, and be adapted to the diopters of the observer. The two eyepieces can be separated from each other to match the distance between of the eyes of the observer.
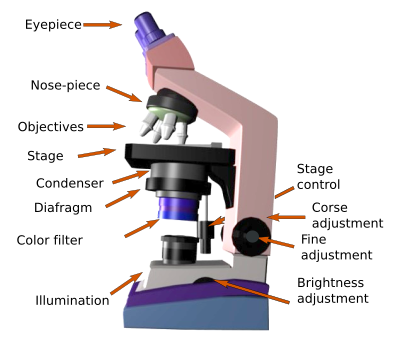
Objective lenses. They are one of the most important part of the microscope because they are the first lenses that gather the light that passed through the tissue. Current microscopes have a nose-piece with several objectives, each with a different magnification power. The more frequent magnifications are: 4x, 10x, 20x, 40x and 100x. By rotating the nose-piece, the objective can be selected and therefore a particular magnification for studying the tissue. Besides magnification, objectives have other features to improve the quality of the image. They can be achromatic, apochromatic, contain fluorite, flat field feature to prevent peripheral curvatures, or do interference contrast to increase border contrast.
When the 100x objective is used, a small drop of a specific type of oil, called immersion oil, is placed between the objective and the coverslip (or the sample). Visible light refraction is high in the air and results in image alterations when observing at high magnifications. Immersion oil minimizes the light refraction, rendering sharp images (Figure 2).

The stage is the platform where the slide with the sample is placed. It has a device to hold the slide and another to move the slide manually and horizontally.
The condenser is a light concentrating lens that focuses the light coming from the lamp into the sample.
The diaphragm is placed between the lamp and the condenser. It increases the contrast of the image and the field depth, that is, the distance in the Z axis of the sample that looks sharp or is in focus.
The lamp is the light source. The light goes through the tissue section and is gathered by the objectives. At the beginning of the microscopy, Sunlight was used, which was focused on the tissue with concave mirror lenses. Nowadays, electric lamps are used with a light beam that is adjusted with a diaphragm and a condenser before hitting the tissue (Figure 3). The light intensity can be selected by a brightness control dial.

Fine and coarse adjustment (or fine and gross adjustment) are intended to get the sampadjustment (or fine and gross adjustment) are intended to get the sample in focus by changing the distance between the sample and the objective lens. It is done by either rising or lowering the stage where the sample is laid on. The distance depends on the objective: shorter as magnification is higher. It also depends on the specs of the objective. Fine and gross adjustment means the amplitude of the distance that can be set with each of them.
2. Modifications
Phase contrast. It is a modification of the standard light microscopy by using special objectives that take advantage of the very small variations in phase when the light goes through tissular structures with different densities. These small differences are translated into changes in the light amplitude, which can be visualized as variations in the image contrasts. Then, different tissular structures are visualized more or less brilliant. Phase contrast microscopy can be used to observe non-stained samples, aqueous solutions, and cell cultures.
Dark field. Dark field microscopy can replace phase contrast microscopy to visualize unstained samples or aqueous samples. Dark field microscopy includes a dark disc under the condenser, between the lamp and the condenser. The dark disk lets pass only the more lateral light, which hits the sample in an oblique direction. Only the light scattered by the sample will be gathered by the objectives. Those areas without tissue are black and different tissue densities reflect a variety of light intensities.
Differential interference contrast (DIC) or Nomarski microscopy. This type of microscopy is present in the most advanced microscopes and needs special objectives and filters. It enhances the contrast of the sample, provides a 3D-like appearance to tissues, and increase the field depth (thickness of the sample in focus). It is based on polarizing filters that let pass the light vibrating in a particular plane. Then, polarized light goes through a glass prism that distribute the light in rays separated by a distance similar to the resolution power of the objective. There is a prism for each objective. After that, the light reaches the sample and each ray takes different paths in the sample because of tissue densities (edges of cells, intracellular densities, extracellular matrix fibers), and the rays change in phase compared to the others, resulting in brightness changes. There is another filter between the objective and the eyepieces to modulate the overall luminosity.
3. Other types
Stereo microscope. It is also known as dissecting microscope. It is commonly used for manipulate small samples and to visualize features that do not need large magnifications, about 7x to 40x, although 100x can be reached. Samples can be visualized at different magnifications by using a zoom system or changing objectives, which is less common. Samples can be studied tridimensionally since stereo microscopes are binoculars. In addition, they have a wide focal distance, that is, there is a long distance between the sample and the objective that makes much comfortable to manipulate samples. Illumination of the sample may come from different sources and angles. Light sources can be external lamps.
4. Fluorescence
Fluorescence microscopy is used to visualize fluorochromes, fluorescent chemical compounds. Fluorescent molecules are able to be excited by electromagnetic radiation with a particular wavelength and emit electromagnetic radiation with other wavelength, usually in the visible light spectrum. Fluorochromes are used to visualize tissue structures, and are commonly used conjugated with antibodies during immunofluorescence protocols. The more popular fluorochromes are excited by ultraviolet light, commonly produced by mercury lamps, and emit in the visible light range. Each fluorochrome is excited by a narrow wavelength range, which is selected by special filters placed between the lamp and the sample. It is possible to excite several fluorochromes present in the sample by using a number of filters with different specs.
More than one fluorochrome, in the same sample at the same time, can be visualized with fluorescence microscopy. It can be done if the absorption wavelength of each fluorochrome are not overlapped (Figure 4). Otherwise, they cannot be distinguished between each other.
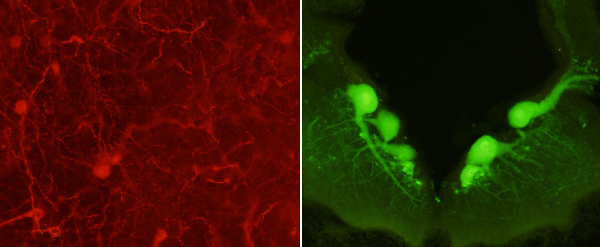
Confocal microscopy is performed by an advance fluorescence microscope that is able to reduce light diffusion from out-of-focus excited fluorochromes. Thus, confocal microscopes get sharper images from different depths in the sample. Furthermore, they are usually connected to a computer so that the digital images obtained from different depths of the sample can be rendered in 3D images.
 Visualization
Visualization