Here, we are dealing with techniques that include chemical reactions involving molecules of the tissue (immunohistochemistry and lectin histochemistry are described in the next pages). The goal of the histochemistry techniques is to detect specific molecules in tissue sections, and therefore it is possible to study their distribution "in situ", that is in the tissue. These molecules can not be readily distinguished by general staining techniques. The tissue has to be treated to reveal the molecule we are interested in. Histochemical techniques can be divided in two groups: chemical reactions and histo-enzymology.
1. Chemical reactions
Chemical reactions are modifications of tissular molecules that allow them to be colored. There are histochemical procedures for staining carbohydrates, proteins and nucleotides. PAS (Periodic Acid Schiff) is the most popular histochemical technique for detecting free or conjugated carbohydrates that can be visualized when they are relatively abundant in the tissue (Figures 1 and 2). The chemical modification consists in the oxidation by periodic acid of close carbon links that have hydroxyl groups. This reaction forms aldehyde groups that are recognized by the Schiff reactive, providing a brilliant red color. Schiff reactive contains pararosaniline (a component of the basic fuchsin), which has been previously treated with sulfuric acid. PAS technique is able to discriminate different types of carbohydrates by adjusting the procedure.


1. Enzymatic reactions
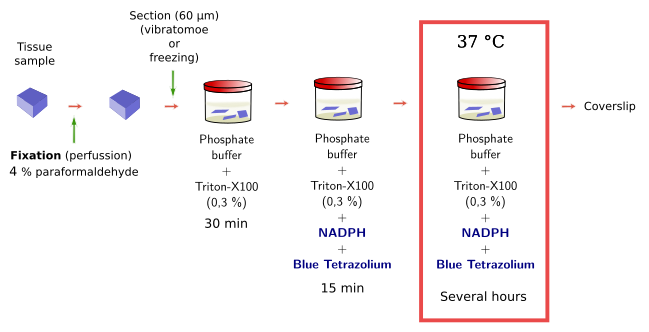
Histoenzymology, or enzyme histochemistry, is based on the capacity of some enzymes to keep their activity after the tissue fixation. These enzymes, and the cells they are located in, can be visualized after the conversion of soluble and colorless substrates to insoluble and colored products by the enzyme activity. Substrates are specific for the enzyme and the products precipitate in the place where the enzyme is. There is a diversity of enzymes that can be detected with this method, such as peroxidases, phosphatases, dehydrogenases, diaphorases, acetylcholinesterase, and some others. It should keep in mind that fixation and embedding of the tissue may affect the enzyme activity. Embedding should be avoided because dehydration and high temperature may damage the enzyme and therefore its activity. That is why histoenzymology is mostly done on freezing or vibratome sections, where no embedding is needed.
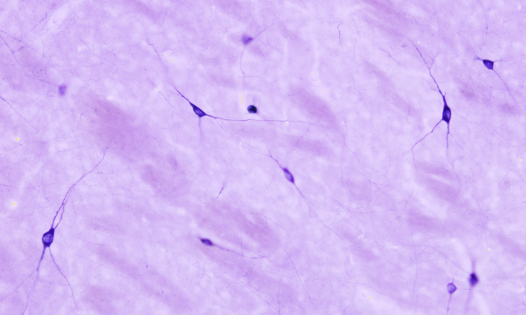
The NADPH diaphorase activity is performed in the nervous system by the endothelial and neuronal nitric oxide synthase enzymes. Nitric oxide has been involved in the control of the blood flow and neuronal activity. By using histoenzymology, neurons (Figure 3) and endothelial cells expressing these enzymes can be quickly and easily identified in tissue sections. The enzymatic reaction is: NADPH + nitroblue tetrazolium = NADP+ + formazan. Formazan is the colored and insoluble product of the reaction that can be observed at light microscopy. An interesting property of formazan is some electron-density, so it can be also observed at transmission electron microscopy.
 General staining
General staining