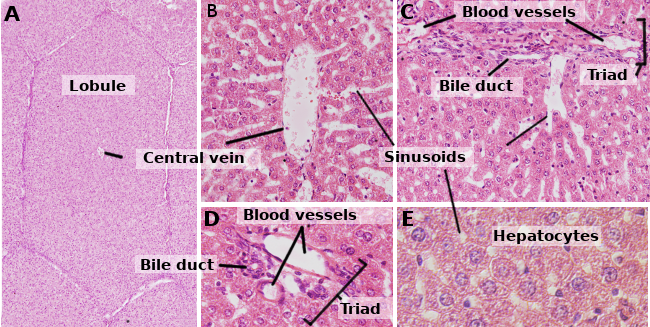
Hepatocytes are the cells that form the liver, accounting for about 80 % of this organ. They are organized in sheets of one cell in thickness. The sheets are connected between one another, forming a spongy-like structure (Figures 1 and 2). Hepatocytes are rather long-living cells, since they are renewed about every 5 months. However, it may change under regenerative processes, where hepatocytes show a high ability for proliferation and regeneration of the damaged liver tissue.

1. Morphology
Hepatocytes are polyedral cells, that is, they have several faces. They usually show 6 faces, but the number may vary. Faces are in contact with either other hepatocyte or with a sinusoid (Figure 3). Hepatocytes are large cells, about 20 to 30 µm in diameter. They usually show a round nucleus centered in the cytoplasm. However, in the liver of adult humans, up to 25 % of the hepatocytes may be binucleated (Figure 2). The majority of nuclei are tetraploids, so they contain double amount of DNA than an ordinary cell. The size of nuclei is variable, although they are larger in tetraploid cells. Nuclei show scattered heterochromatin and one or more nucleoli. In normal conditions, it is not common to observe mitotic hepatocytes (1 every 10000 or 20000 hepatocytes). However, the mitotic hepatocytes increase enormously under liver injuries and regeneration processes. The cytoplasm features vary according to the physiological state of the cell, mostly influenced by fat and glycogen depots. There are many small mitochondria, 800 to 1000 per hepatocyte. It is estimated that one hepatocyte may contain about 50 Golgi apparatuses that are usually organized in stacks of 3 to 5 cisterns showing thickened lateral regions containing dark bodies. Cistern stacks are somehow scattered in the cytoplasm, although they are often observed close to bile canaliculi (Figures 3 and 4). Hepatocytes contain many peroxisomes (200 to 300), more than other regular cells. Near the bile canaliculi, many lysosomes are also found.


Abundant glycogen and lipid depots are found in the hepatocyte cytoplasm (the granular appearance of hepatocytes after haematoxylin and eosin staining is due to the holes left by lipid extraction from the cytoplasm during the tissue processing). In the cytoplasm, there are also residual bodies containing lipofuscin. The smooth endoplasmic reticulum is quite abundant, although its size varies with the metabolic activity of the hepatocyte. It is concentrated around glycogen depots. Within a liver lobule, there are morphological differences when comparing peripheral and central hepatocytes, mostly influenced by the blood irrigation features. For example, after digestion, peripheral hepatocytes are the first to store glycogen, but they are the last to mobilize this glycogen when the rest of the body is demanding it. However, fat storing happens first in the centrally located hepatocytes, which usually have more abundant smooth endoplasmic reticulum. On the other hand, the rough endoplasmic reticulum shows 50 % more surface in the peripheral and medially located hepatocytes than those in the inner part of the liver lobule.
Unlike other epithelial cells, hepatocytes are not attached to a basal membrane. Their basolateral membranes are surrounded by low-density extracellular matrix synthesized by the hepatocytes themselves. It facilitates the diffusion and exchange of molecules with the sinusoids through the space of Disse, or perisinousoidal space, which is the space between the fenestrated endothelium and the hepatocytes. This extracellular matrix lacks laminin, at least when the hepatocyte is differentiated. However, laminin, type IV collagen and fibronectin are thought to be necessary for a proper hepatocyte differentiation. Hepatocytes are connected between each other by gap junctions, and attached by adherent junctions, desmosomes and tight junctions.
Hepatocytes are polarized cells, that is, there are differences between the regions facing the bile canaliculi and the regions close to the sinusoids (Figure 3). The polarity is essential for a correct functioning of the hepatocyte, and it is disorganized in many liver pathologies. The apical region is in contact with the bile canaliculi. As in the apical domain of many epithelial cells, there are tight junctions in the apical domain of hepatocytes, that in this case seal and maintain the integrity of the bile canaliculi. The apical membrane is folded into microvilli that enormously increase the membrane surface. The apical plasma membrane is about 13 % of the total hepatocyte membrane, and it is able to have a large amount of molecules. Removing tight junctions from the apical domains leads to cell polarity disorganization. Hepatocyte polarity and bile canaliculi are established during the embryo development period.
The functional polarity relies on an unequal distribution of transporters and other membrane molecules between the apical and the baso-lateral plasma membrane domains. For example, ABC transporters (ATP binding cassettes) are among the most important apical transporters in hepatocytes. The Golgi apparatus, endosomes and cytoskeleton (microtubules and actin filaments) are responsible for the differential distribution of molecules between the two membrane domains. There are two main delivering pathways of proteins to the apical domain (Figure 5). a) From the Golgi apparatus, the proteins (for example, ABC transporters) are sent in vesicles toward the apical plasma membrane or toward recycling endosomes, which function as intermediaries between the Golgi apparatus and the plasma membrane. b) Other proteins follow the transcytosis pathway, traveling first to basolateral membranes, then they are enclosed in endocytosis vesicles toward endosomes, where they are again packaged in vesicles and shipped to the apical plasma membrane. More rare is a pathway involving the exocytosis of lysosomes, for example cooper transporters are shipped in this way.

2. Functions
The main function of hepatocytes is to metabolize substances coming from digestion. The liver is irrigated by the portal vein that gathered molecules resulting from digestion in the intestine. Hepatocytes are also strongly involved in detoxification of potentially harmful molecules. On the other hand, hepatocytes synthesize bile, which is finally released into the intestine and helps in digestion. For metabolizing molecules from digestion, hepatocytes are placed in a privileged location within the liver: in contact with sinusoids, which bring digested molecules. Their plasma membranes also form the bile canaliculi that drain the bile from the lobules of the liver.
Glucose levels. Hepatocytes fetch glucose molecules coming from digestion and store them as glycogen, which is mobilized when the body needs energy. Glycogen is commonly found near the endoplasmic reticulum, since the glucose-6-phosphatase enzyme is located in this organelle. Glucose-6-phosphatase catalyzes glucose-6-phosphate, the molecular form of glucose after glycogen catabolism, and produces free glucose, which can exit the hepatocyte and reach the blood stream.
Molecular synthesis. Bile salts, that help with the digestion of fat, are synthesized by the hepatocytes. In the smooth endoplasmic reticulum, there are many enzymes involved in the synthesis of cholesterol and other lipids. In addition, hepatocytes produce lipoproteins needed for the transport of lipids in the blood stream. Fibrinogen for blood clotting and plasma albumins are also synthesized by hepatocytes. In the liver, the urea is produced as a byproduct of the protein degradation. The production and accumulation of high amount of urea in the organism may be harmful. Hepatocytes also store vitamin A and B, and heparin.
Lipid metabolism. Beta-oxidation, involved in lipid catabolism, is carried out in the many peroxisomes of hepatocytes.
Detoxification. Hepatocytes gather nutritious substances coming from digestion, but they are also the firsts to receive potentially toxic substances. Ethanol of alcoholic beverages is mainly degraded in the liver, actually in the peroxisomes of the hepatocytes. Half of the ingested alcohol is transformed in acetaldehyde in these organelles. There are enzymes in the smooth endoplasmic reticulum involved in the degradation or inactivation of toxins and drugs. During the periods of high demand of toxic substances removal, like during medicine treatments or continuous alcohol drinking, the endoplasmic reticulum may become the largest organelle of the hepatocyte. Drugs are usually inactivated by conjugation with other molecules. For instances, glucosyltransferase conjugates molecules with barbiturates.
Store and regulation of iron. Hepatocytes may store iron, which is concentrated in cytoplasmic depots as iron bound to ferritin. Hepatocytes may capture iron in several ways: bound to transferrin, as part of heme groups and from non-heme groups. Transferrin bound-iron enter the cell by TRF1 receptor mediated endocytosis. When endocytic vesicles fuse with endosomes, transferrin releases Fe3+, which is transformed to F2+ and the extruded to the cytosol by DMT1 (Divalent Metal Transporter 1). Heme-iron molecules are also endocyted and translocated to the cytosol through the endosomal membrane by the HRG1 transporter. However, the majority of the iron enters from the extracellular space through the ZIP14 transporter placed in the hepatocyte plasma membrane facing the sinusoids. Once in the cytosol, the iron is bound to ferritin and stored in the cytoplasm because the free iron is toxic. Releasing the iron from the enterocyte is mediated by the ferroportin transporter found in the plasma membrane near the sinousoids.
After the bone marrow, the liver is the second major production center of heme groups. Heme group is a prosthetic group (non peptidic) present in several proteins for transporting oxygen, and in those enzymes like catalases and peroxidases that protect against oxidant substances. It is also part of the mitochondrial and peroxysomal cytochromes. The higher amount of heme groups is found in the hemoglobin, which are synthesized in the bone marrow. In the liver, heme group synthesis depends on the amount of microsomal p450 cytochrome required by the cell, so that most of these heme groups are part of p450 cytochromes.
Hepatocytes release the hepcidin hormone, which regulates the systemic iron concentration in the body. This hormone controls the amount of iron in the plasma by favoring the internalization and degradation of ferroportin, which is an iron transporter found in enterocytes, macrophages and hepatocytes. Removing ferroportin inhibits the release of iron from these cells. Hepcidin synthesis is regulated by the transferrin-iron concentration in the plasma, by iron depots in the hepatocytes and by inflammation. The erythropoyetic activity inhibits the release of hepcidin.
-
Bibliography ↷
-
Gissen P, Arias IM. 2015. Structural and functional hepatocyte polarity and liver disease. Journal of hepatholoty. 63: 1023-1037.
Knutson MD. 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature review in molecular cell biology. 15:19-33.
Weiss L, Greep RO. 1982. Histología. 4ªedición. Editorial el Ateneo. Barcelona.
-