The primary root is an essential component of the root system. It influences the final organization of roots both in morphology and length. Roots grow in length by the activity of the root apical meristem, which is protected by the root cap. Proliferation and differentiation of the root apical meristem give rise to cells that are first organized in tissues, forming the so-called primary root. This is the primary growth. The primary root shows a less complex organization than the stem because it lacks nodes, internodes, and leaves. This organization is supposed to be ancestral, that is, more similar to the first land plants.
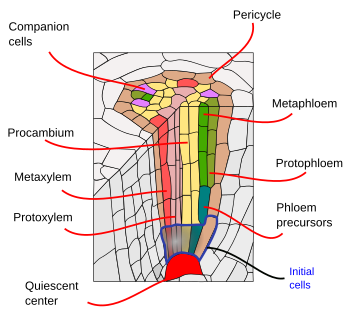
The root apical meristem consists of a quiescent center, surrounded by the initial cells. The quiescent center is the niche for the initial cells, which are the stem cells (Figures 1, 2 and 3). Initial cells give rise to progenitor cells that proliferate, grow in size and differentiate into the variety of functional cells that form the primary root tissues. All tissues emerge toward the opposite site of the root tip, including the lateral cells of the root cap. Only the cells of the columnella, the central cell rows of the root cap, are generated toward the tip. The columnella contain the cells that sense gravity, known as statocysts, driving in this way the direction of the root elongation. Progenitor cells proliferate in a root segment just above the initial cells referred to as the proliferation zone, which is transformed into in the elongation zone when progenitors cells stop dividing and increase their sizes. Finally, after reaching a particular size, cells differentiated in the differentiation or maturation zone.

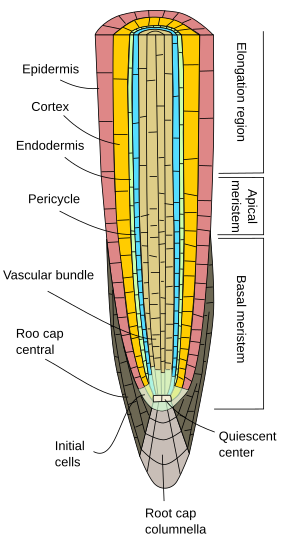
No matter the plant group, the primary root consists of the epidermis, or rhyzodermis, which is generally uniseriated (one cell-thick layer), lacks stomata, and shows root hairs in the maturation zone (Figures 1, 2, and 3). The epidermis is exposed to the environment and interacts with fungi and bacteria to form mycorrhizae and nodes to fetch nitrogenous and phosphorous substances. In general, the epidermal cells near the tip show a very thin cuticle, or it is absent, facilitating in this way an incoming flux of water and minerals. The epidermis is also a protective tissue. There are examples, such as xerophyte plants or roots near the ground surface, where a cell layer of cells with suberized cell walls is found just below the epidermis. This layer is called the hypodermis, and it can further specialize into the exodermis. The exodermis can be found below the root epidermis of many angiosperms. The morphology and function of the exodermis are similar to those of the endodermis (see below), for it works as a barrier against the free diffusion of molecules, regulating the water and mineral fluxes in and out of the root. Exodermis cells show suberin layers in their cell walls and Casparian bands on the radial and transversal cell wall surfaces. Along the exodermis layer, there are some cells lacking suberin and lignin; they are "windows" through which lateral roots cross in their way to the exterior. In those plants living more than one year and that do not show secondary growth (see next page), a thick cuticle is developed by epidermal cells, which indicates a protection role, while in other species the epidermis is shed out and substituted by an exodermis with lignified and suberized cell walls, maintaining the cells alive.
Root hairs absorb water and minerals. They are elongated epidermal cells found in the maturation region that increase the absorption surface in contact with the external environment and, therefore, the absorption capability. Root hairs are continuously formed and disappear as the root grows because the maturation region follows the growth of the root tip at a more or less constant distance. The number of root hairs is about 20 to 500 per cm2 in the roots of trees and about 25000 per cm2 in the winter rye. The number may also change depending on the soil and environmental conditions.
There are three root hair organization patterns (Figure 4). The density of root hairs depends on the environmental conditions. For example, in low-phosphate soils, the number of root hairs is higher to increase the total root absorption surface. Many symbiotic organisms, such as nitrogen-fixing bacteria, are associated with root hairs.

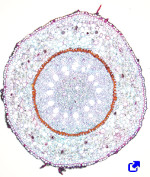
The root cortex is found below the epidermis, or hypodermis (Figure 5). In roots, the cortex is thick or very thick (much more than in stems), and it is made of parenchyma cells generally specialized in storing, mostly starch. The storage function is well developed in carrots and beets. In these species, even the secondary xylem can work as a storage tissue. The cortical parenchyma of roots may become a photosynthetic parenchyma in aerial roots. It often contains large intercellular spaces to facilitate root aeration. In aquatic plants or plants living in waterlogged soils, the intercellular spaces are really enlarged, and the parenchyma is called the aerenchyma, which allows the supply of oxygen from the stem to the roots. The water absorbed by the root hairs and epidermis also circulates (via apoplastic) through the intercellular spaces of the cortical parenchyma. In addition, parenchyma cells are connected through plasmodesmata, which are pathways (via simplastic) for water and minerals that connect adjoining cells. In the root cortex may be colenchyma and sclerenchyma, which are often observed in the periphery. The mutualistic symbiont relationship with fungi (mycorrhiza) is established by parenchyma cells.


A feature of root primary growth is the endodermis, the innermost layer of the cortex. This layer can also be found in some leaves and stems. The endodermis is evolutionary conserved, from ferns to angiosperms. It is observed in early primary root development and performs its function in the root hair region. The endodermis is a one-cell-thick layer of tightly compacted cells with cell walls partially impregnated with suberin and forms lignified thickenings known as the Casparian stripes. The cell compaction and the impermeability of the Casparian stripes make water and solved substances cross the endodermis through the cytoplasm of endodermal cells (the simplastic pathway) to reach the vascular bundles. The cell membrane is strongly adhered to the cell wall, just below the Casparian stripes. Hence, endodermis is a barrier against free diffusion that controls the substances traveling from the soil to the vascular bundles and from here to the rest of the plant body. In those root regions with secondary growth, both endodermis and cortex are shed out (see next page).
The suberin lamellas of the endodermis are an evolutionary feature of seed plants, while they are absent or independently acquired by ferns and lycophytes. The suberin layers can be induced by hydric stress or low nutrients under abcisic acid action. The number of suberin layers is higher in the more mature regions of the primary root. It is thought that they work as barriers that prevent the loss of water that was uptake in the more young regions of the root, where suberin layers are not present.
Casparian stripes are primary wall structures that encircle the endodermis cells as a belt arranged longitudinally. They are not secondary cell walls. The stripes are continuous between neighboring cells through the middle lamella. In 3D view, they are organized as a cylindrical fishing net, where the ropes are the Casparian stripes and the holes are the endodermis cells. Casparian stripes contain lignin. Suberin can be found in the cell wall of endodermis cells after the Casparian stripes are formed. Suberin is laid as layers over the cell wall surface, with different thicknesses depending on the cell localization. For example, those endodermis cells found closer to the xylem poles do not synthesize suberin. They are called passage cells. The ultrastructure of the endodermis cell wall is similar in many plant species, and only the thickness and number of the suberin layers may change. In some plant species, endodermis undergoes an additional developmental stage where their cell walls become lignified and Casparian stripes show a U shape. Passage cells, however, keep thin cell walls. The continuous growth of roots led to the death and disappearance of endodermis. In pteridophytes, seedless plants, and some grasses, the endodermis may work as meristematic tissue and give rise to lateral roots.
Below the endodermis, there are one or two layers of parenchyma cells with very thin cell walls, referred to as the pericycle. Pericycle cells can restart the meristematic activity and form lateral roots. In the older part of the roots, pericycle cells are sclerified. In plants with secondary roots, the pericycle contributes to the formation of the vascular cambium and phellogen (cork cambium).
The vascular bundles, xylem and phloem, are found in the inner part of the root. Primary xylem and primary phloem are arranged in separate and alternate rows in a radial way. According to the number of rows, there are diarch (2), triarch (3), and tetrarch (4) (Figure 6). Tetrarch organization is typical of dicotyledons and gymnosperms. Monocotyledon adventitious roots are polyarch, with many rows of xylem and phloem circularly arranged. The number of rows is a feature of the species, but not always. The center of the root is occupied by primary xylem and phloem, except in some species that show a medulla free of vascular tissue. In the primary root, the protoxylem is found at the tips of the ribs of the metaxylem. It is difficult to distinguish the protophloem from the metaphloem in the roots. f the metaxylem. It is difficult to distinguish the protophloem from the metaphloem in the roots.

Lateral roots are generated after the embryonary period and determine the root system morphology of the plant. The lateral root formation begins in the pericycle near the apical tip of the root, in the region where the cells become differentiated. This is different from how the stem branches and leaves are formed since they emerge from superficial meristems in an exogenous way. The location of the starting point of a lateral root is influenced by the organization of the vascular bundle. Lateral root primordia are formed at points opposite to the xylem poles in dicotyledons or to the phloem poles in some monocotyledons. In some species, the endodermis also collaborates in the formation of the lateral roots.
Adventitious roots emerge after plant germination from cells near the vascular bundles, either as a normal process or after an inductive process. They can be generated in stems, leaves, and roots.
The water absorption region of the primary root is where root hairs are found, which, compared with the length of the root, is proportionally small. That is why most plants are helped by fungi. Mycorrhizae are mutualist symbiotic associations between plants and fungi (mostly ascomycota and basydiomycota). It is thought that up to 95 % of the land plant species show mycorrhizae. The fungi provide water and minerals, while the plant supplies organic molecules from photosynthesis. There are two types of mycorrhizae: endomycorrhizae and ectomycorrhizae. Ectomycorrhizae form a dense net of hyphae around the root tip. Some of them enter the root by crossing the epidermis and occupying the intercellular spaces of the cortical parenchyma, forming the so-called Harting net. It is more often, however, the endomycorrhiza association, where the hyphae do not form a peri-root net, but they enter the root and proliferate in the cortical parenchyma. Hyphae cross the cell wall and ramify between the cell wall and the plasma membrane, forming a kind of arbuscle. That is why endomycorrhizae are known as arbuscular mycorrhizae.
-
Bibliography ↷
-
Bibliography
Furuta KM, Hellmann E, Helariutta Y. 2014. Molecular control of cell specification and cell differentiation during procambial development. Annual review of plant biology. 65: 607-638.
Peret B, De Rybel B, Casimiro I, Benkova E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. 2009. Arabidopsis lateral root development: an emerging story. Trend in plant science. 14: 399-408.
Salazar-Henao J, Vélez-Bermúdez IC, Schmidth W. 2016. The regulation and plasticity of root hair patterning and morphogenesis. Development. 143: 1848-1858.
-