Once the tissue is fixed, it is further processed for observation with the microscope. This usually means making sections first for staining and then observation. As a general rule, samples are hardened before sections are obtained. The thinner we want the sections, the harder has to be the sample. Tissues are already hardened by fixation, but not so much, so that sections thicker than 20-30 µm are difficult to obtain. The fixative provides enough hardening if we want thick sections (between 30 and 200 µm). Devices like vibratome can get thick sections directly from only fixated tissues, and it is used when a very good molecular preservation is required. However, thinner sections are often required, so the sample must be harder. There are two common procedures to do this: freezing and embedding (Figure 1).
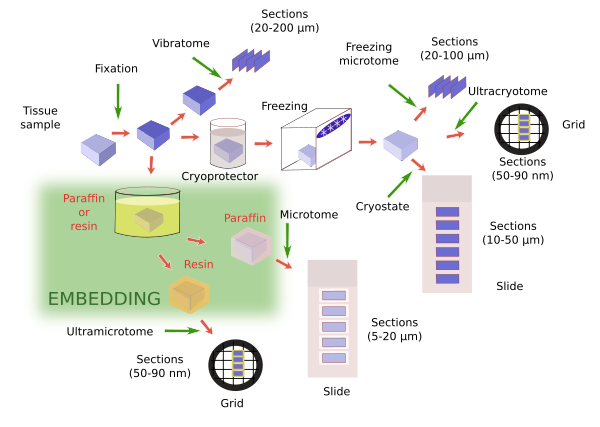
Freezing of previously fixated tissues provides proper hardening for obtaining sections with thickness ranging from about 50 µm to tens of nanometers (nm). Several sectioning apparatuses are used: freezing microtome for thick sections (larger than 20 µm), cryostat for thin sections (from 5 µm to about 20 µm) and ultracryotome for ultrathin sections (tens of nm). Freezing forms water crystals that can destroy the tissues. This damages can be lessen. a) Immersion of samples in cryoprotecting solutions that decreases the size of crystals during freezing. Sucrose (30%) is the most common cryoprotector, but dimethyl sulfoxide, glycerol, ethylene glycol are also used. They are chosen depending on the type and size of the sample, and the procedure to be later performed. b) Another important parameter is the freezing speed. The quicker the sample is frozen, the smaller the water crystals, so it minimizes the tissue damages. For example, freezing in liquid nitrogen is frequently used. In practice, both treatments are consecutively used in the same protocol. For example, crytoprotection and fast freezing are common steps for electron microscopy studies.
Embedding is the most widely used method for hardening samples. During embedding, substances in liquid state are infiltrated through the sample and, after a period of cooling or polymerization, the embedding substance, and therefore the sample, gets solid without altering the morphological or molecular features of tissues. In this way, depending on the embedding substance, very thin section (from tens of µm to tens of nm) can be obtained without breaking or spoiling the tissue. In addition, embedding is a good method for preserving samples during long periods of time. A number of embedding substances are available to get specific section thickness and for performing particular techniques. For light microscopy studies, paraffin is the most common embedding substance. For electron microscopy, epoxy and acrylic resins are the most used embedding medium. There are other embedding substances, such as celoidin, nitrocellulose, polyethylene glycol and wax polyester.
The majority of embedding media are not hydrophilic, i.e., they are not miscible with water. It means that the water of the sample should be exchanged for hydrophobic compounds, and then the embedding media must substitute the compound. If some water is remaining in the sample, the embedding process does not properly reach all the sample regions, so that the technique yields poor-quality sections and tissues.
 Fixatives
Fixatives