1. Selection
- Pinocytosis
- Receptors
2. Types
- Clathrin
- Caveolae
- Non-coated
- Macropinocytosis
- Phagocytosis
Getting extracellular molecules into the cell is essential for cell survival. Endocytosis is a mechanism to get into the cell a large amount of molecules. By endocytosis, molecules and particles are enclosed by plasma membrane, that, once detached from the plasma membrane, form membrane bound compartments within the cell. These compartments are mostly vesicles, but also larger compartments. It is the opposite process to exocytosis, which is the fusion of vesicles with the plasma membrane for releasing their content into the extracellular environment.
Besides assimilating molecules targeted for degradation, endocytosis performs other functions. Together with exocytosis, it is a mechanism for renewing the plasma membrane. Molecules are removed from the plasma membrane as part of the endocytic vesicle membrane or compartment. At the same time, endocytosis balances the addition of membrane to the plasma membrane carries out by exocytosis. In this way, the amount of plasma membrane remains stable and functional.
1. Selection of molecules
There are three ways for molecules to be endocytosed: nonspecifically as soluble molecule or pynocytosis, specifically recognized and linked to a membrane receptor or receptor mediated endocytosis, and as part of the membrane of the endocytic vesicle or membrane bound compartment (Figure 1).
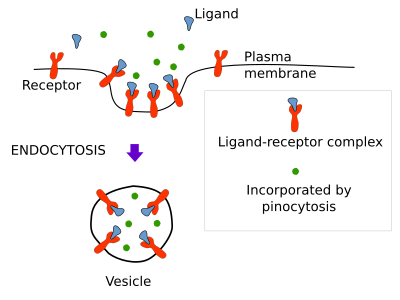
Pinocytosis
Pinocytosis is the nonspecific incorporation of extracellular soluble molecules into vesicles or larger membrane bound compartments. It is clear that vesicles, or other type of membrane compartments, carry molecules in solution when detached from the plasma membrane toward the inner cytoplasm. So, all endocytic processes carry molecules by pinocytosis. However, the amount of soluble material is particularly important in a type of endocytosis known as macropinocytosis (see below), which, as its name suggest, is specialized on fetching large amount of soluble extracellular molecules.
Receptor mediated endocytosis
Receptor-mediated endocytosis captures specific extracellular molecules by means of receptors located in the plasma membrane. More than 25 receptor types have been found involved in this type of endocytosis. Molecules and small particles, which may be at low concentrations in the extracellular space, are efficiently included into vesicles by this mechanism. Molecules (also known as ligands) are recognized by these receptors, and the ligand-receptor complexes gather in small plasma membrane areas where vesicles are going to be formed. After pinching off from the plasma membrane, vesicles containing a high concentration of ligands are moved away from the plasma membrane. For example, cholesterol enters the cell by receptor-mediated endocytosis as part of low-density lipoproteins (LDL). LDL contains many cholesterol molecules surrounded by a lipid monolayer and a protein. When a cell needs cholesterol, or there is a high concentration of cholesterol in the blood, many receptors for LDL are synthesized and moved to the plasma membrane. There, receptors bind LDL particles, receptor-LDL complexes move by lateral diffusion and are eventually trapped by incipient membrane invaginations. Once formed, vesicles are targeted to endosomes, where LDL particles are released from their receptors. After that, LDL particles are moved to the lysosomes for degradation and cholesterol is released. When this pathway is damaged, for example, by lacking LDL receptors or by failures on the receptor-LDL recognition, cholesterol accumulates in the blood and may cause arteriosclerosis and heart attack.
2. Types
Several types of endocytosis have been described depending on the vesicle or compartment size, the material to be incorporated, and the mechanism of vesicle formation. Here, we will deal with the following types of endocytosis: clathrin coated vesicles, caveolae, non-coated vesicles, and macropinocytosis. Phagocytosis is being included here too, although it is a particular mechanism for capturing large particles such as bacteria, viruses, and cellular fragments, which are first recognized by receptors and then surrounded by membrane (Figure 2).
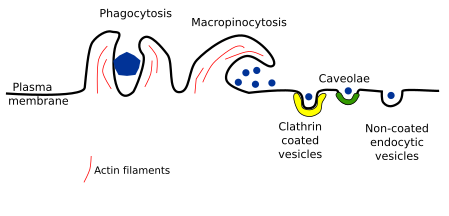
Clathrin coated vesicles
Endocytosis mediated by clathrin coated vesicle is the main mechanism for incorporating integral proteins and lipids, both located in the plasma membrane, as well as extracellular molecules with a size smaller than 156 nm, including some viruses. Clathrin coated vesicles are assembled in plasma membrane areas where the cytoplasmic protein clathrin is present, which alternates between the cytosol and the plasma membrane. Clathrin coated vesicles are initiated in specific regions of the plasma. For instance, those enriched in PI(4,5)P2. In fibroblasts, these areas may be up to 2 % of the total surface of the plasma membrane. Clathrin shows a three arms molecular structure, and when these proteins gather together are able to assemble into a regular pentagonal net. This organization and the way they assemble help in the membrane invagination and final closure of the vesicle. The clathrin assembling produces vesicles of about 120 nm in diameter. Clathrin, however, is not in direct contact with the plasma membrane. There area about 50 different proteins involved in the formation of the vesicle, all of them coming from the cytosol. Between the clathrin net and the plasma membrane, these proteins participate in many aspects of the vesicle formation. For instance, adaptor proteins are involved in selecting the receptors, and therefore the cargoes, to be included in the vesicle. Clathrin coated vesicles can transport many types of cargoes.
The plasma membrane curvature to form vesicles is driven by the clathrin coat, actin filaments and excision proteins. Actin filaments seem to participate in later stages, when invagination of the membrane is already started, and stop collaborating when the vesicle is formed. Once the vesicle pinches off, clathrin coat is disassembled and the "naked" vesicle is targeted to specific organelles within the cell, usually to early endosomes.
Caveolae
Caveolae were described in the 1950s by P. Palade after observing animal tissues at transmission electron microscopy. They are small invaginations (45-80 nm) of the plasma membrane that can be observed in most eukaryotic cells. It is supposed that most caveolae become vesicles (but see below). Caveolae are abundant in endothelial cells, muscle cells and adipocytes. Caveolae membrane contains the protein caveolin, as well as other glycosylphosphatidylinositol-linked proteins, and is enriched in sphingolipids (sphingomyelin and glycosphingolipids) and cholesterol. The expression of caveolin in a cell is enough to form caveolae. There are around 100 to 200 caveolin molecules in one caveola and different types of caveolin. Caveolae may be observed in the Golgi apparatus as well, so it has been suggested that vesicles formed from caveolae may work as transporter of certain molecules between the plasma membrane and the Golgi apparatus. However, most vesicles coming from the plasma membrane caveolae are fused with early endosomes. Some authors suggest that these endosomes are different from other early endosomes, and the name caveosome has been suggested.
Caveolae functions are not clear yet. Although a mayor role in endocytosis has been suggested, there is no strong experimental support. Other suggested functions are modulating signal transduction by gathering receptors of the plasma membrane, such as tyrosine kinase receptors. They may also participate in the lipid traffic between the plasma membrane and some organelles. They even may be involved in fighting some tumors. Choleric toxin, folic acid, and other molecules enter the cell by means of caveolae.
Non-coated vesicles
This type of endocytosis has been proposed because endocytic vesicles are still formed when both clathrin and caveolae endocytic pathways are inhibited. Furthermore, invaginations and vesicles show a slightly different morphology. Some toxins, such as cholera toxin, enter the cell within this type of vesicle. It is largely unknown how is the mechanism of vesicle formation and how transported molecules are selected, but lipid rafts may have some role here. It is also unknown whether this endocytic pathway is regulated or not.
Macropinocytosis
This endocytic mechanism allows the cell to fetch a large amount of extracellular material. At the surface of the cell, the plasma membrane, together with underlying cytoplasm, can protrude forming large waves arranged circularly. The crests of these waves fuses together or fall onto the plasma membrane, that results in a large membrane compartment inside the cell known as macropinosome. This mechanism relies on actin filaments and myosin motor proteins. Macropinocytosis is not just for feeding, as in amoebas, but also is useful for recycling plasma membrane. During cell movement, it is suggested that macropynocitosis facilitates the transport of large amounts of membrane from the rear of the cell to the front part. Furthermore, some bacteria are able to enter the cell by inducing macropinocytosis, avoiding in this way the phagocytic degradation pathway.
Phagocytosis
Phagocytosis is a type of endocytosis that engulfs large particles, like bacteria, cellular leftovers, and viruses. Phagocytosis is carried out by specialized cell, such as macrophages, neutrophils, and dendritic cells. For instance, macrophages are responsible for removing immunoglobulins bonded to other particles or pathogens like viruses and bacteria. They also remove thousands of red blood cells every day. Macrophages are resident cells in different tissues and sometimes are called with different names. For example, they are the Kupffer cells in the liver and the microglia in the brain. Protozoa, however, use phagocytosis for feeding. It is plausible that phagocytosis has evolved from a feeding mechanism in unicellular organisms to a defense role in multicellular animals.
Phagocytosis begins with the specific recognition of the particle by plasma membrane receptors of the macrophage. Molecules exposed at the surface of the particle or pathogen, such as phosphatidylserine in apoptotic cells and immunoglobulins and complement proteins of the immune system, are recognized by these receptors. If enough amount of receptors is linked to their ligands, they gather together, and laminar and pseudopod protrusions are emitted from the cell surface to engulf the particle. However, if the number of receptor-ligand complexes is low, phagocytosis does not proceed. On the other hand, macrophages membrane harbor a diversity of receptors having different affinities for different molecules that work together to trigger the engulfment of the particle.
Actin filaments and myosin motor proteins generate cell protrusions during phagocytosis. The cell expansions surround the particles, fuse their ridges and form an inner membrane bound membrane called phagosome, which mature and fuse with lysosomes for particle degradation.
-
Bibliography ↷
-
Bibliography
Bloomfield G, Kay RR. 2016. Uses and abuses of macropinocytosis. Journal of cell science. 129: 2697-2705.
Cheng JPX, Nichols BJ. 2016. Caveolae: one function or many? Trends in cell biology. doi: 10.1016/j.tcb.2015.10.010.
Mayor S. Pagano RE. 2007. Pathways of clathrin-independent endocytosis. Nature reviews in molecular and cell biology. 8:603-612.
Watanabe S, Boucrot E. 2017. Fast and ultrafast endocytosis. Current opinion in cell biology 47:74-71.
-
 Exocytosis
Exocytosis 