1. Mecanotransduction
Cell attachment to the extracellular matrix or to neighboring cells by adhesion molecules, such as integrins, cadherins, selectins or immunoglobulins are not simply for getting attached or for resisting compression or stretching forces. These proteins do not only have a role with only mechanical consequences, they also act as mechanoreceptors. Having both an extracellular and an intracellular domain (Figure 1), which are connected to each other. Therefore, they can exchange information, that is, they allow communication between extracellular and intracellular environments (Figure 2). When they bind their extracellular “ligands”, their cytosolic domains can trigger internal processes, thus affecting the physiology of the cell. They can interact with some intracellular signaling pathways, affect cell mobility, modify the gene expression, influence the cell cycle, and even affect cell survival. Also, malfunctions of cell adhesion may cause numerous pathologies in organisms, which can be lethal in some cases. In fact, most cells do not differentiate, proliferate or survive if they are not properly adhered. Metastasis requires a previous change in cell adhesion of tumor cells. Hence, a flow of information from outside the cell is transmitted to the cytoplasm thanks to adhesion proteins, which is a mechanism similar to that of the classic receptors of the plasma membrane. That is, it is important for cells to be attached and to know what type of molecules they are attached to.


On the other hand, changes in cellular physiology may affect cell adhesion. A flow of information is then produced in the opposite direction, that is, certain cytosolic molecules can affect the intracellular domain of the adhesion proteins, which in turn change the affinity for their extracellular ligands (Figure 2). In this way, adhesion molecules behave as classic receptors or, according to many authors, can even be considered as true receptors since they establish a bidirectional way of communication between extracellular and intracellular environments. For example, the modulation of many internal cell signaling processes have been assigned to integrins, a repertoire of processes so broad that they can be compared to some classical receptors of the plasma membrane. Regardless of the direction, the information is transmitted between domains by conformational changes in the three-dimensional structure of the transmembrane adhesion protein.
Cells can also modulate their adhesion status by changing the number and type of adhesion molecules that are present in the plasma membrane. These two variables, number and type, are controlled by cells according to their physiological needs.
Let's see some examples in which adhesion molecules affect cellular physiology and how cells modify the properties, type and number of adhesion molecules to perform some functions:
2. Cell movement
When a cell is about to move, it has to change its adhesion status. It means breaking the adhesion with the adjoining cells and extracellular matrix to which it is attached to, and synthesize new adhesion molecules that let the cell moves away through the tissue. For example, embryonic cells migrate through the embryo and occupy new places to form new organs by changing their adhesion capabilities. This occurs frequently in the epithelia, where cells must lose their polarity, detach from neighboring cells, become migrating cells and travel to their new destination. The target area is also recognized by adhesion. A similar mechanism has been proposed for cancer cells when they became metastatic. Cells do not swim, but crawl by establishing adhesion points with the environment, i.e., the extracellular matrix and other cells. The adhesion points work as anchor points to pull on the rest of the cell. Thus, adhesion molecules are indispensable elements at the leading edge of the moving cell, or front of the cell, because they anchor the cell and allow the cytoskeleton to pull the rest of the cell forward (Figure 3).


Cadherins are involved in the loss of adhesion affinity between neighboring cells (Figure 4). Epithelial cells, for instance, express E-cadherins, whereas mesenchymal cells, which usually move, express other cadherins like N-cadherin, R-cadherin and cadherin-11. N-cadherin favors cell movement and it has been shown that the expression of N-cadherin leads to a shift from cellular immobility to mobility. In fact, there is a relationship between the progression of cancer and the cadherins that are expressed in cancer cells. For example, cancer cells that do not express N-cadherins are not metastatic, but those expressing this cadherin are. Metastasis can be induced experimentally by inducing the expression of N-cadherin. Besides adhesion, N-cadherins, but not E-cadherins, produce other intracellular effects that favor cell mobility. Thus, when N-cadherin binds its ligand, its extracellular domain interacts with the extracellular domains of growth factor receptors. This prevents the elimination of growth factor receptors from the plasma membrane and, therefore, favors cell survival. N-cadherins also promote the survival and growth by the inactivation of apoptotic pathways. These pathways, which lead to cell death, are usually activated when cells lose their adhesion points.
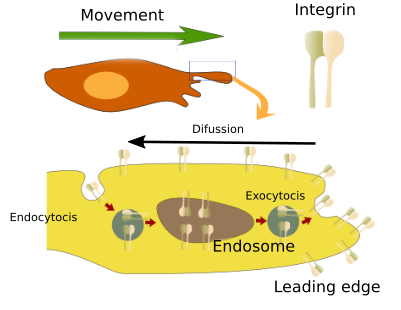
The change of adhesion molecules, which is important to initiate cell movement, is also needed for cell traveling through the tissue. It has been reported that a turnover of integrins and cadherins is required in the plasma membrane of the leading edge of moving cells. This turnover is mediated by early endosomes and other organelles involved in vesicular trafficking (Figure 5). Vesicular traffic of integrins and cadherins promotes the invasion of tissues during cancer cell metastasis, probably increasing the replacement and recycling of focal adhesions, which are points of attachment. This mechanism also works in normal cells that move in the tissues. Previously, it was thought that endocytosis carried integrins from the rear of the cell to the leading edge to supply adhesion molecules to cell extensions (podia) that were projected toward the direction of movement. However, it seems that local vesicular traffic and rapid recycling the integrins in the area of the leading edge prevents these molecules from diffusing through the rest of the plasma membrane of the cell, so they concentrate in the front of the cell, where they must perform their function. This mechanism is vital for the leading edge to explore, adhere, and drag the rest of the cell. In addition, cell movement is helped by the action of cadherins.
The movement of a cell population, which occurs during embryonic development and during wound repairing, is a frequent phenomenon based on cell adhesion. Although tight junctions, as well as adherent junctions and desmosomes, are required to preserve the integrity of the epithelia, only adherent junctions are necessary for coordinated cell movements. Adherent junctions are cell-cell junctions allowing a cytoskeleton wiring that extends throughout the cell population and makes cells behave in a coordinated way (Figure 6). For example, covering a wound with epithelium. In cell population movements, actin-myosin complexes are important. The driving forces are generated by myosin II, while the polymerization of actin filaments allows the extension of the cytoplasm of the cells leading the front of the population. Adherent junctions connect the actin network of the entire cell population so that the tensile force of one cell is directly transmitted to the other cells.

3. Changes in gene expression
The binding of an adhesion protein to its ligand can to change gene expression (Figure 7). Many cytosolic proteins can interact with the intracellular domains of adhesion molecules. These proteins, which are intermediaries between adhesion molecules and the cytoskeleton, can enter the cell nucleus and behave as transcription factors or modulate the activity of transcription factors, changing the expression of some genes. They have amino acid sequences that are recognized by importins and exportins. Importins and exportins carry proteins into the nucleus or outside the nucleus, respectively, by interacting with the nuclear pore complexes. However, when intermediary proteins bind to the cytosolic domain of adhesion molecules, recognition amino acid sequences are hidden.
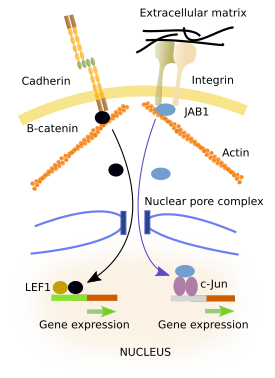
JAB is a protein that can be found either anchored to the cytosolic domain of integrins or in the nucleus (Figure 7). The location of JAB depends on whether integrins are bound to its ligand or not. Inside the nucleus, JAB activates c-jun transcription factors, which promote gene expression. β-catenin can be linked to the cytosolic domain of E-cadherins (Figure 7). β-catenin can enter the nucleus where it gets associated with LEF-1 leading to the expression of some genes. It seems that the number of β-catenin linked to E-cadherins does not depend on the binding state of E-cadherins, but on the amount of E-cadherins located in the membrane, which in turn depends on the general level of adhesiveness and the state of differentiation of the cell. A large amount of E-cadherin molecules in the plasma membrane indicates that the cell is strongly attached, and a high amount of the total cellular β-catenin is linked to them, i.e., in the cytoplasm. Thus, less β-catenin is left to enter the nucleus. ZO-1 is a protein that can be associated with tight junctions. It can be released from tight junctions and transferred to the nucleus. Inside the nucleus, it affects the expression of certain genes, which seems to be necessary for the reorganization and differentiation of epithelia.
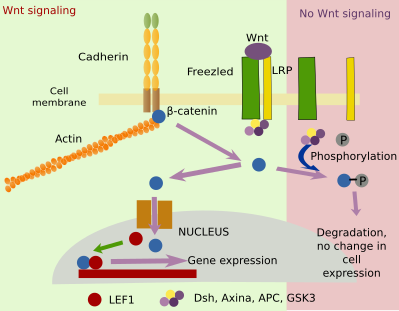
Adhesion molecules and their associated proteins can influence some cellular signaling pathways. Cadherins themselves can interact with the extracellular domains of growth factor receptors. β-catenin, protein associated to the cytosolic domain of cadherins, is also regulated by the Wnt signaling pathway (Figure 8). Wnt affects the proliferation, differentiation and homeostasis of cells by activating Frizzled and LRP receptors. Wnt intracellular signaling needs β-catenin to change gene expression. Activation of Frizzled and LRP by Wnt prevents phosphorylation of β-catenin so that β-catenin does not degrade. However,β-catenin availability depends on the number of cadherins. In this way, adhesion and Wnt influence each other through β-catenin.
4. Cell cycle
Progress through the different phases of the cell cycle affects cell adhesion and adhesion affects the way the cell cycle progresses. For example, the binding of integrins to their ligands is necesary for many cells to progress from G1 phase to S phase. The binding of integrins activates intracellular signals that synergistically cooperate with growth factors to promote the cell cycle. On the other hand, the adhesion of the cadherins inhibits the progress of cells to the next phase. In cancer cells, adhesion signaling pathways affecting the cell cycle are inhibited.
The binding of integrins to their ligands causes the activation of intracellular molecules that eventually promote the synthesis and stability ofcyclin D, which is needed for the activity of the cyclin-dependent kinase found in the core of the molecular machinery that allow the progression of the cell cycle forward to phase S. In the case of cadherins, when they are linked to other cadherins of neighboring cells, they also bind α- and β-catenins to their cytosolic domains, which in turn bind actin filaments. When the extracellular domain of cadherins are not linked to other cadhering, β-catenins are released and can travel to the nucleus, as described above. In the nucleus, they promote the expression of cyclin D and other proteins that stimulate proliferation. Integrin and cadherin molecular pathways do not work independently. For example, the integrin pathway activates Src protein, which increases cadherin internalization and degradation.
During mitosis, many cells lose adhesion and become rounded, but cytokinesis and the onset of the phase G1 require cells to be attached again. At the beginning of mitosis, many proteins that interact with the cytosolic domains of adhesion proteins are phosphorylated, which contributes to decrease the cell adhesion. These phosphorylated proteins move to other parts of the cell, where they perform functions related to mitosis. Cytokinesis requires the action of integrins because if they are inactivated there is no the separation of the daughter cells. Both cadherins and integrins have been involved in settling the position of the cytokinesis furrow that ultimately separates the two cells. They are also involved in asymmetric divisions (when the two daughter cells are not equal).
Bibliography
Aplin AE, Juliano RL. Regulation of nucleocytoplasmic trafficking by cell adhesion receptors and the cytoskeleton. The journal of cell biology. 2001. 155:187-191.
Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion.. Trends in cell biology. 2008. 18:257-263.
Gordon MD, Nusse R. Wnt signalling: multiple pathways, multiple receptors, and multiple transcription factors. The journal of biological chemistry. 2006. 281:22429-22433.
Hervy M, Hoffman L, Beckerle MC. From the membrane to the nucleus and back again: bifunctional focal adhesion proteins.Curren opinion in cell biology. 2006. 18:524-532.
Hynes RO. Cell adhesion: old and new questions. Trends in cell biology. 1999. 12:M33-M37.
Ladoux B, Mege RM. Mechanobiology of collective cell behaviours. 2017. Nature reviews in molecular cell biology. 18:743-757.
Pugacheva EN, Roegiers F, Golemis EA. Interdependence of cell attachment and cell cycle signaling.Current opinion in cell biology. 2006. 18:507-515.
Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. The journal of cell sciences. 2008. 121:727-735.