1. Morphology
2. Stratum basale
3. Stratum spinosum
4. Stratum granulosum
5. Stratum corneum
6. Functions
Keratinocytes are the main cellular components of the epidermis. They release very little extracellular matrix so that the plasma membrane of adjoining keratinocytes are very closeto one another. The cohesion of keratinocytes is strengthened by many desmosomes that maintain the integrity of the epidermis. Keratins, a type of intermediate filament of the cytoskeleton, form the major family of proteins synthesized by keratinocytes. Keratinocytes go through a characteristic life cycle that begins in the basal layer of the epidermis and ends in the more superficial surface, or free surface, of the epidermis.
1. Morphology and differentiation
The morphology of keratinocytes changes during their life cycle, that lasts about 1 month in humans. The changes are progressive from the basal layer, where keratinocytes are born, until the superficial layer, where they die and detach from the epidermis. The morphological differences of keratinocytes lead to the features of the epidermal layers. The layers or strata (stratum in singular) are the stratum basale, stratum spinosum, stratum granulosum and stratum corneum. Excepting the stratum basale, which is relatively constant, the layers may change in thickness depending on the body region (Figure 1). Thus, the summed thickness of the epidermis, i.e., the thickness of the strata together, may range from 50 µm in body regions with little mechanical stress to 1 mm in those regions like the palms and the soles, where friction is higher.

2. Stratum basale
The stratum basale, or germinative layer, (Figures 1 and 2) is a one thick cell layer made up of keratinocytes, and other very scarce cells, found in the deeper part of the epidermis. Stratum basale keratinocytes are attached to the basal lamina by hemidesmosomes. These connections helps to maintain the integrity of the epithelium and controls the proliferation and differentiation of keratinocytes.

Keratinocyte stem cells are found in the stratum basale. They are rounded to columnar in shape, about 6-10 µm in size, and show more affinity for basic dyes than differentiated keratinocytes because of their high content in ribosomes. The stem cells of interfollicular epidermis (outside of the hair follicles) differentiate into keratinocyes, but not in other epidermal cell types. There are also keratinocyte stem cells in the hair follicles that differentiate into cells of the hair follicle (keratinocytes and sebaceous glands), but also in keratinocytes of the interfollicular epidermis.
Proliferation and differentiation rates of keratinocyte stem cells and the replacement rate of mature keratinocyte need to be synchronized in the epidermis. The replacement rate depends on circumstances like wound repairing, rubbing, or organ growth. The contact of the stem cells with the basal lamina seems to be important for regulating this proliferation rate. For example, when integrins, adhesion proteins that attach keratinocytes to the basal lamina, loose contact with the basal lamina, stem cells begin to differentiate and migrate to the upper layers of the epidermis.
Keratinocytes of the stratum basale synthesizes part of the basal lamina. Integrins that attach keratinocytes to the basal lamina are also involved in maintaining the structural integrity of the basal lamina.
3. Stratum spinosum
The stratum spinosum is found just above the stratum basale (Figures 2 and 3). It is made up of more or less polyhedral keratinocytes of about 10 to 15 µm in size, larger than those in the stratum basale, showing more eosinophilic cytoplasm and one or two clearly visible nuclei. Many keratin bundles are present in the cytoplasm, known as tonofilaments, that joint together to form tonofibrils. Tonofibrils can be observed at light microscopy and contribute to the cytoplasmic staining with eosin. In this stratum, keratinocytes are called spinous keratinocytes because they share many desmosomes (Figure 4) and adherent junctions. These cell-cell junctions are radially distributed in the plasma membrane and are connected by tonofibrils. At light microscopy, the cell junctions look like spines, therefore the name of this layer.

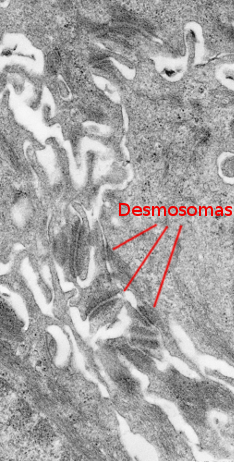
Keratinocytes express the intermediate filament keratins in the cytoplasm. The type of keratin expressed changes as keratinocytes move toward the upper surface of the epithelium. In the more superficial part of the stratum spinosum, keratinocytes become more flattened, probably induced by the progressive change in the type of synthesized keratins.
The stratum spinosum is thicker in those regions of the skin exposed to rubbing or mechanical stretches. The strong cell-cell adhesion in this stratum helps get epidermis more resistant .
4. Stratum granulosum
In the outer layers of the stratum spinosum, keratinocytes change the gene expression and produce kerato-hyalin granules, therefore forming the stratum granulosum (Figures 2 and 3). The stratum granulosum is relatively thin, about 15 µm, and keratinocytes are flattened and show many kerato-hyalin granules in the cytoplasm. These granules are polygonal, non-delimited by membrane, and highly basophilic. The keratin filaments progressively grow and are more abundant, which makes stronger the epidermal barrier. These cellular features are the starting point for cornification or keratinization of keratinocytes.
Lamellar bodies are found in the stratum granulosum. These cellular structures are not present in the stratum basale, and start to be visible in the upper stratum spinosum. They are very abundant in the stratum granulosum. Lamellar bodies are organelles, about 100 to 300 nm, made up of stacks of lipid layers, so they show a lamellar structure. Their function is performed in the stratum corneum and is related to the detached cell process in this stratum and with the impermeability of the epidermis. Lamellar bodies are exocyted and their components are modified extracellularly to form lipid layers parallel to the epidermis surface.
The nucleus and organelles of cells in the stratum granulosum are degraded, while the cytoplasm is nearly full of keratin filaments. The permeability to calcium and other ions is increased during the last stages of cell differentiation. Ions boost the activation of transglutaminases and leads to the formation of a protein structure below the plasma membrane known as the cell envelope. This structure grows in thickness as keratinocytes degenerate, and it is important for organizing the keratin filaments parallel to the epithelial surface and to combine these filaments with the extracellular lamellar bodies.
Tight junctions are formed between keratinocytes of the stratum granulosum and make a sealed barrier that can be crossed only by small molecules and ions. Keratinocytes die by a process known as cornification, and become a cornified anuclear cell. Cornification is a mechanism different from apoptosis. Before dying, keratinocytes synthesize proteins and lipids that contribute to the formation of the superficial barrier of the epidermis.
The stratum lucidum is a transitional layer between the stratum granulosum and the stratum corneum. It can be visualized with the light microscope. It is found in skin regions with strong mechanical stress, like the soles.
5. Stratum corneum
The stratum corneum is made up of degenerated keratinocytes, the so-called corneocytes (Figures 2, 4, and 5). Corneocytes are connected by desmosomes (corneosomes) to one another and embedded in an extracellular matrix enriched in non-polar lipids organized in layers. During the last stages, corneocytes loss their nucleus, get full of keratins, and are surrounded by a layer of proteins, as well as a covering of chemically bound lipids.

The thickness of the stratum corneum is variable and depends on the general thickness of the epidermis. Thus, the more keratinocytes are produced in the stratum basal, more thickness is observed in the stratum spinosum and corneum. The dead of cells in the stratum granulosum leads to the progressive loss of desmosomes along the stratum corneum, which makes cell-cell junctions weaker and therefore cells and cell fragments detach from the epidermis. Filaggrin and keratin account for 80 to 90 % of the proteins of the stratum corneum.
This layer protects from drying, pathogens, and chemical and physical damages. The processing of the lipids of the lamellar bodies is important for the epidermis. For example, glycerol is formed from lamellar bodies and helps the epidermis to maintain hydration . Free lipids of the surface contribute to keep the epidermis a bit acid, with pH around 5 in humans. Moreover, cholesterol sulfate is transformed in cholesterol, which participates in the detachment of the more superficial layers of the stratum corneum. Released lipids are organized in several sheets that make possible impermeability of the epidermis.
6. Functions
Keratinocytes form the epidermis of the skin, and their main function is to organize a barrier between the external and the internal environments. They protect against mechanical damages, ultraviolet light, pathogens and noxious chemical substances. It is very important the role of the epidermis to prevent the water lose. In addition, keratinocytes are very active during repairing processes after a wound.
Water loss barrier
Water loss prevention mostly relies on the stratum corneum. It is like a wall with bricks and mortar, where the bricks are cells and the mortar is the extracellular lipid aggregates.
Barrier against pathogens
The epidermis is the first physical hurdle that pathogens need to overcome in order to enter the organism. In addition, keratinocytes release cytokines that inhibit and neutralize pathogens. After a wound or damage, keratinoncytes stimulate inflammation and Langerhans cell activation, which are the antigen presenting cells of the epidermis.
Skin pigmentation
The skin pigmentation is intended for protection against ultraviolet light. In mammals, melanin is the pigment that makes skin darker. Melanin is synthesized by melanocytes, a very scarce cell type found scattered in the stratum basale of the epidermis and in the hair follicles. Melanin is packaged in cytoplasmic organelles called melanosomes. The production and release of melanosomes are influenced by environmental variables as well as by paracrine substances released by keratinocytes. Before they are released, melanosomes are distributed through the melonocyte cytoplasm and cell processes that extend among the surrounding keratinocytes. After a high melanin synthesis, melanosomes are exocyted from melanocytes and captured by neighbor keratinocytes. It is estimated that each melanocyte provides melanin to about 45 keratinocytes. Once melanosomes have been incorporated in the keratinocytes of the stratum basale, they are transported toward the upper epidermis strata as loaded keratinocytes leave the stratum basale and move to form the upper strata. In this way, all epidermis strata have melanin, even the stratum corneum. Altogether, the color of the skin depends on the amount of melanin that melanocytes and keratinocytes contain.
Wound repairing
After a wound, keratinocytes change from a steady state to another where they begin to move. Disconnection from the basal membrane mediated by loss of desmosomes and change in the expression of integrins, , as well as the response to cytokines released by other cells, boost the keratinocytes motility. In this way, keratinocytes may move to and seal the damaged areas. .
-
Bibliography ↷
-
Eckert RL, Rorke EA. 1989. Molecular biology of keratinocyte differentiation. Environmental health perspectives 80:109-116.
Grinnell F. 1992. Wound repair, keratinocyte activation and integrin modulation. Journal of cell sciences 101:1-5.
Madison KC. 2003. Barrier Function of the Skin: "La Raison d’EŒtre’" of the Epidermis. The journal of investigative dermatology 121: 231-241.
Nemes Z, Steinert PM. 1999. Bricks and mortar of the epidermal barrier. Experimental and molecular medicine 1: 5-19.
Proksch E, Brandner JM, Jensen J-M. 2008. The skin: an indispensable barrier. Experimental dermatology 17: 1063–1072.
Simpson CL, Patel DM, Green KJ. 2011. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nature reviews in molecular and cell biology 12:568-580.
Yamaguchi Y, Brenner M, Hearing VJ. 2007. The Regulation of Skin Pigmentation. Journal of biological chemistry 282:27557-27561.
-