1. Morphology
2. Fusion and fission
3. Functions
- ATP
- Metabolism
- Others
4. Protein import
5. Renewing
Mitochondria are almost ubiquitous organelles in eukaryotic cells. They show a double membrane, they can grow and divide, and two mitochondria can fuse to form a new one, so they show a changing morphology. Mitochondria are organelles that evolved by endosymbiosis from an ancestral alfa-proteobacterium that was included in an archaean linage. The two cells end up as eukaryote cells. The main function of mitochondria is the synthesis of ATP as a source of energy for the cell. However, a number of other functions are also performed by mitochondria.
1. Morphology
The shape of mitochondria is variable, from long and branched to small ellipsoids. It could be said that there are mitochondria as individual organelles and as a dynamic mitochondrial networks. As a networks or isolated, mitochondria are made up of an outer membrane, an inner membrane, intermembrane space and the mitochondrial matrix enclosed by the inner membrane (Figures 1 and 2).
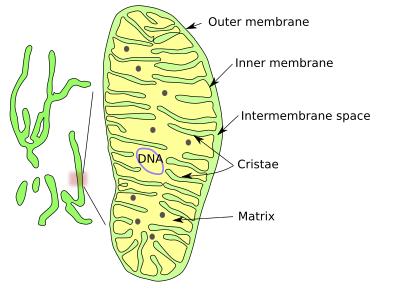
The outer mitochondrial membrane is highly permeable and contains many copies of the protein aquaporin, which is an aqueous channel. This membrane is a kind of sieve, allowing the pass of all those molecules smaller than 5000 Dalton, including small proteins.
On the other hand, the inner mitochondrial membrane is highly impermeable to ions and small molecules. Mitochondria have to make the inner membrane impermeable enough to form a stable proton gradient. Mitochondria synthesize cardiolipin, a phospholipid with highly unsaturated fatty acid chains that is translocated to the inner membrane, providing both hydrophobicity and fluidity (mitochondria lack cholesterol).
The Inner membrane is heavily folded with invaginations toward the matrix. Each fold is called a mitochondrial crista (cristae in plural). There are three morphological types of cristae: discoidal, tubular and flattened. Cristae form a distinct compartment in the inner membrane because they contain a particular set of proteins. The number and form of cristae is the result of mitochondrial activity. Functional protein complexes of the respiratory chain and ATPases are moved to cristae, as are proteins for assembling iron-surfur complexes. Cristae are actually a way to increase the membrane surface to accommodate many respiratory chain complexes and ATPases. In a hepatic cell, the inner mitochondrial membrane is about 1/3 of the total cell membrane.
DNA, ribosomes, and many enzymes are located in the mitochondrial matrix, where several metabolic pathways take place. The mitochondrial DNA is located in places referred to as nucleoids, which may contain more than one DNA molecule. Nucleoids are attached to the inner membrane by MitOS complex proteins. DNA contains about 16500 paired bases and around 37 genes that, in humans, code for 13 proteins. Hundreds of copies of the mitochondrial DNA can be found in one cell. Mitochondrial DNA replication is not coupled to the cell cycle, and it can be replicated at any time in the life of a cell.
Mitochondria, or parts of the mitochondrial network, are moved through the cytoplasm by the cytoskeleton. They show an extraordinary motility and can be moved to energy- or calcium-demanding regions of the cell (see below). The movement is not continuous but saltatory, which means that it is stalled from time to time. Long-distance movements are ruled by microtubules, whereas local movements depend more on actin filaments. Within the neuronal axons, the speed of the mitochondrial movement along microtubules is around 0.1 to 1.4 µm/s.
Mitochondria communicate with each other by releasing molecules, through membrane contact sites, and by fission and fusion. There are also narrow membrane nanotubules that transiently connect nearby mitochondria.
2. Fusion and fission
Mitochondria often divide and fuse with each other, mixing their mitochondrial DNA. If two cells are fused, the mitochondrial network becomes homogeneous in 8 hours. Fusion and fission of mitochondria are complex mechanisms because the two mitochondrial membranes are involved, and they must be opened and sealed correctly. The number of mitochondria in a cell may change because of this behavior. However, the mitochondrial volume in a cell seems to be related to the cell volume.
The presumed functions of fusion-fission are to share the synthesized molecules with distant regions of the mitochondrial network, minimize local damages, and share the DNA. There is evidence that mitochondrial fusion is boosted when the cell is under moderate stress, so it has a protective role.
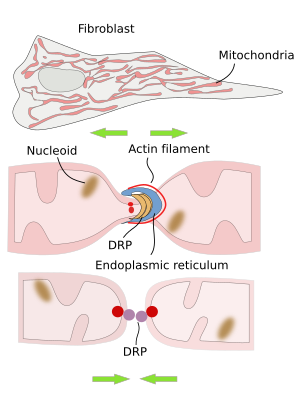
The division of mitochondria is mediated by DRP proteins (dynamin related proteins) (Figure 3). In mammal cells, the endoplasmic reticulum is found in the region where mitochondrial division is going to happen. Before the split and after recruiting DRP proteins, there is an initial constriction of the mitochondria produced by actin filaments. The role of the endoplasmic reticulum appears to be related to the lipid transfer between the endoplasmic reticulum and mitochondrial membranes. This lipid exchange seems to be important for synthesizing some lipids like phosphatidylethanolamine and cardiolipin in mitochondria.
3. Functions
One of the most important functions of mitochondria is synthesizing ATP, the fuel for most cellular processes. Another function is β-oxidation, which is part of fatty acid metabolism. They also work as compartments for calcium storage, the formation of iron-sulfur groups, the synthesis of amino acids, and hemo groups. Mitochondria are involved in apoptosis, cancer, aging, and pathologies like Parkinson's disease and diabetes. On the othe hand, comparative studies of the mitochondrial DNA are very useful in anthropology for deciphering genealogies and in other phylogenetic studies because mitochondrial DNA is inherited by maternal lineage and recombination does not occur in mitochondrial DNA during sexual reproduction.
ATP synthesis
Most ATP in eukaryotic cells (in non-photosynthetic eukaryotes) is synthesized in mitochondria. The citric acid metabolic cycle uses acetyl-CoA to produce CO2 and NADH. Electrons from NADH are transferred to the electron transport chain of proteins located in the cristae membrane. Electrons are finally transferred to oxygen to form water. The transport process of electrons through this chain of proteins is able to move protons across the cristae membrane, from the mitochondrial matrix to the cristae inner space. A gradient of protons is generated, which is used by the ATP synthase to produce ATP. ATP synthase is a transmembrane protein located in the cristae membrane. This metabolic process is referred to as oxidative phosphorylation because the oxygen is the last electron acceptor, and it chemically joins inorganic phosphorus and ADP into ATP. The many copies of these enzymes and protein complexes make up to 80 % of the membrane weight.
The set of proteins that carrie out the transport of electrons is known as the electron transport chain or respiratory chain (Figure 4). It is made up of around 40 proteins, 15 of which are for electron transport. Most of these proteins are grouped into three complexes: the NADH dehydrogenase complex, the b-c1 cytochrome complex, and the cytochrome oxidase complex. All have mechanisms to extrude protons from the mitochondrial matrix to the cristae inner space across the inner mitochondrial membrane, a process coupled to the transport of electrons.
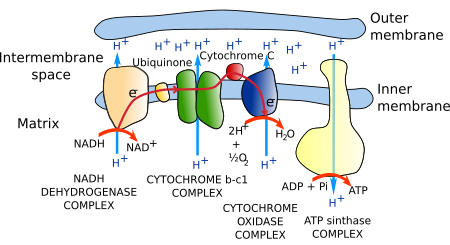
The whole process is able to generate a concentration of protons ten times higher in the inner space of the cristae than in the matrix. In addition, a more negative charge environment is generated in the mitochondrial matrix as a consequence of proton extrusion. In this way, protons are pushed by the electrochemical gradient to enter the mitochondrial matrix again.
ATP synthesis is not the only purpose of the proton gradient. Other electrically charged molecules, such as pyruvate, ADP, and inorganic phosphorus, must be pumped into the mitochondrial matrix, whereas ATP, which is released into the mitochondrial matrix, must be transferred to the cytosol. Pyruvate and inorganic phosphorus get into the matrix, coupled to the inward flux of protons by symport transport. However, ADP enters coupled to the ATP exit by antiport transport.
Lipid metabolism
A significant amount of cell lipid synthesis takes place in the mitochondria. They synthesize lysophosphatidic acid, from which triglycerides are synthesized. Phosphatidic acid and phosphatidylglycerol can also be synthesized in mitochondria. Phosphatidylglycerol is needed for the synthesis of cardiolipin and phosphatidylethanolamine.
Others
In some species, mitochondria have evolved into organelles with particular functions. For example, hydrogenosomes are related to hydrogen metabolism and mitosomes are involved in sulfur metabolism. These organelles do not contain DNA. Recently, mitochondria, together with the endoplasmic reticulum, have been involved in the generation of peroxisomes.
During cellular stress, mitochondria may participate in the stress responses to counteract the stress effects or damages. However, if the stress is long-lasting or too intense, the cell triggers a regulated cell death mechanism known as apoptosis. Mitochondria are key organelles for apoptosis. Apoptosis may also be activated in physiological and morphogenetic processes.
4. Protein import
Mitochondria have a very low number of genes compared to the large number of different types of proteins they contain. A yeast mitochondria contains around 1000 different proteins, whereas it contains around 1500 in humans. Only a few proteins are coded by mitochondrial DNA, and most mitochondrial proteins have to be imported from the cytosol. Importing proteins is a complex process since there are several compartments in the mitochondria where proteins can be targeted: external membrane, intermembrane space, inner membrane and mitochondrial matrix. The imported proteins contain amino acid sequences that are like a mailing address, informing mitochondrial transporters where these proteins have to be targeted.
5. Mitochondrial renewing
The mitochondrial population is steadily renewing by removing and formation of mitochondria. New mitochondria can only be formed by splitting existent mitochondria and can be removed by macroautophay (specifically known as mitophagy), a cellular mechanism to remove large amounts of cytoplasmic content.
-
Bibliography ↷
-
Friedman JR, Nunnari J. 2014. Mitochondrial form and fucntions. Nature. 505: 335-343.
Kiefel BR, Gilson PR, Beech PL. 2006. Cell biology of mitochondrial dynamics. International review of cytology. 254: 151-213.
MacAskill AF, Kittler JT. 2010. Control of mitochondrial transport and localization in neurons. Trends in cell biology. 20: 102-112.
Pickles S, Vigi P, Youle RJ. 2018. Mitophagy and quality control mechanisms in mitochondrial maintenance. Current biology. 28: R160-R185.
-
 Peroxisomes
Peroxisomes