Some techniques specifically detect a molecule or molecular domain by taking advantage of the properties of proteins like lectins and immunoglobulins. Lectins are proteins used to recognize and bind to different types of carbohydrates and carbohydrate moieties. Although there is no chemical modification of tissular molecules, lectin techniques are usually found in the histochemistry sections of textbooks.
Lectins are proteins containing molecular domains that are able to recognize terminal carbohydrates present in olygosaccharide chains. The saccharides may be either free or as part of other molecules like glycoproteins. Thus, lectins recognize glycoconjugates. Lectins can be found in animals and plants with a variety of functions. For example, the exit of lymphocytes from blood vessels is mediated by a type of lectin known as selectin. Endothelial cells near a damaged tissue express selectins in their surface that recognize and link carbohydrates of the lymphocyte surface. Once it is attached to the endothelial surface, the lymphocyte can cross the endothelial layer. In the histology lab, lectins are used to study the distribution of a variety of glycoconjugates. Each lectin specifically recognizes a particular carbohydrate. There are 5 types of lectins that recognize mannose (Man), galactose/N-acetylgalactosamine (Gal/GalNAc), N-acetylglucosamine (GalNAc), fucose (Fuc) or sialic acid (Neu5AC), respectively.
Ccommercially available lectins are named after the animal or plant they are isolated from. The most used lectins and the carbohydrates they recognize are shown in the Table 1.
| Carbohydrate | Scientific name | Acronym | Specific carbohydrate |
| Glucose / Mannose | Galanthus nivalis Canavalia ensiformis Lens culinaris Pisum sativum |
GNA Con-A LCA PEA |
Manα1,3Man > Manα1,6Man > Manα1.2Man αMan > αGlc > GlcNAc αMan > αGlc > GlcNAc αMan > αGlc > GlcNAc |
| N-Acetyl-glucosamine | Griffonia simplicifolia Datura stramonium Tritricum vulgare |
GSA-II DSA WGA |
Terminal α,βGlcNAc, glycogene Galβ1,4GlcNAc(N-acetyllactosamine) > GlcNAc GlcNAc(β1,4GlcNAc)1-2 > β1,4GlcNAc1NeuAc |
| N-Acetyl-galactosamine / galactose |
Dolichus biflorus Helix pomatia Arachis hypogaea Ricinus communis |
DBA HPA PNA RCA-I |
GalNAcα1,3GalNAc > αGalNAc GalNAcα1,3GalNAc > αGalNAc Terminal Galβbe1,3GalNAc Terminal βGal > αGal > GalNAc |
| L-Fucose |
Aleuria aurantia Lotus tetragonolobus Ulex europaeus |
AAA LTA UEA-I |
αL-Fuc αL-Fuc > αL-Fuc1,2Galβ1,4GlcNAc > L-Fucα1,2Galβ1,3GlcNAc αL-Fuc |
| Sialic acid |
Maackia amurensis Sambucus nigra |
MAA SNA |
NeuAcα2,3Galβ1,4GlcNAc NeuAcα2,6Gal = NeuAcβ2,6GalNAc |
Table 1. Common lectins used in histology labs. The carbohydrates they recognize and species they were obtained from are shown, as well as the acronym and the specific carbohydrate for each lectin. The symbol > means decreasing affinity to the right, meaning that a lectin can detect the same carbohydrate with a variety of chemical links, but with different affinity. | |||
The distribution of lectin-carbohydrate binding in the tissue may be studied by using different methods. In the direct detection methods, lectins are conjugated with enzymes, like peroxidase or alkaline phosphatase, or with fluorescent molecules. Once the lectin is attached to the tissue, the activity of the conjugated enzyme can be observed by using specific substrates (histoenzymology), whereas fluorescent molecules can be observed with the fluorescent microscope. In the indirect detection methods (Figures 1 and 2), an interposed molecule is placed between the lectin and the enzyme or fluorescent molecule. Lectins are usually conjugated with biotin, and biotin is detected with avidin-enzyme or avidin-fluorescent complexes (Figure 2). In tissue sections, lectins can also be detected by immunohistochemistry, that is specific antibodies obtained against the lectin.
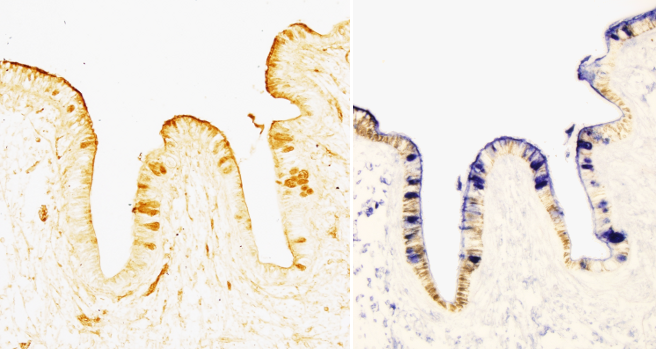
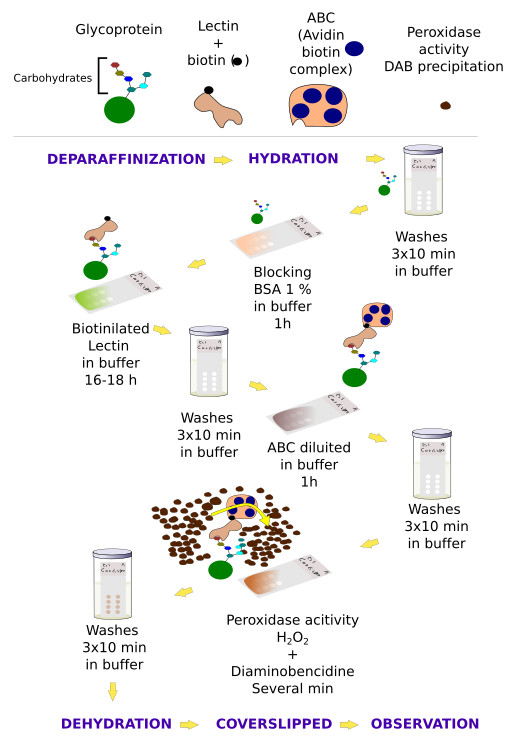
Lectin labeling is usually combined with other techniques that get more information about the carbohydrate composition of tissular glycoconjugates. For example, desulfation uncovers sulfate ester bonds of the terminal chains, and removing beta glycosidic bonds allows knowing if carbohydrate chains are O-linked (attached through an oxygen on a hydroxyl group) or N-linked (attached through a nitrogen) type glycans. Alkalinization removes O-linked bonds.
 Histochemistry
Histochemistry