1. Morphology
2. Organization
- Thin filaments
- Thick filaments
- Other proteins
- Costameres
- Mechanism
3. Innervation
4. Types
Striated skeletal muscle cells (SSMC) form the voluntary contraction muscles, which are usually attached to bones by tendons. However, SSMC not attached to bones can be found. SSMC are actually syncytia, that is, a cytoplasm containing many nuclei. They are very long cells characterized by a highly well-developed cytoskeleton. The cytoskeleton is responsible for the shortening of the cell length, which in turn produces the muscle contraction, and therefore the movement of the organism.
1. Morphology
SSMC are really long cells ranging from several millimeters to more than one meter. That is why they are also known as muscle fibers. In transverse section, they are 10 to 100 µm in thickness. These dimensions are achieved by the fusion of several hundreds of undifferentiated muscle cells, known as myoblasts, and a posterior period of elongation. SSMC contain many nuclei located in the peripheral cytoplasm, just below the plasma membrane or sarcolemma. During differentiation, the nuclei of myoblasts are lined up within the cell and then moved to the periphery where they are immobilized by the cytoskeleton. Many other organelles are also displaced to the cell periphery. These movements leave much room in the inner part of the cell, which can be occupied by the cell contraction machinery (Figures 1 and 2). The plasma membrane of SSMC show many tubular invaginations that form the T-tubules (transverse tubules). T-tubules are found at the level of the Z lines (see below) and are involved in cell contraction.
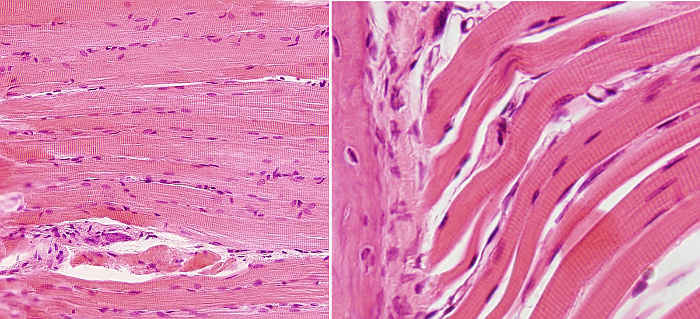
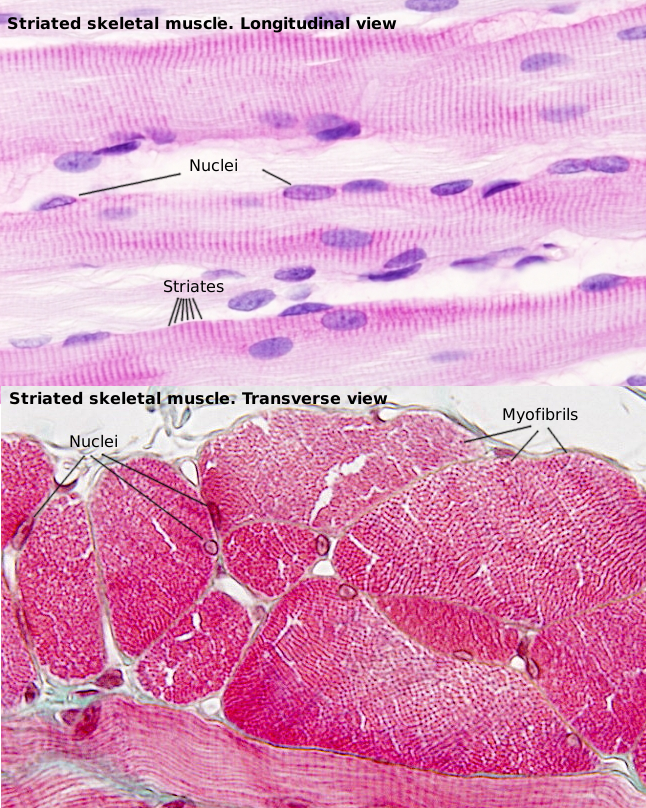
The SSMC content is mostly cytoskeleton, including actin filaments and myosin II filaments, which is a motor protein. Both types of filaments associated to form bundles known as myofibrils. At light microscopy, myofibrils can be visualized as darker dots in transverse sections (Figure 2, see below). Each myofibril is surrounded by membranes of the smooth endoplasmic reticulum, known in SSMC as sarcoplasmic reticulum (Figure 3). The endoplasmic reticulum is organized as a net of tubules around the myofibrils, and this net is also tightly associated to T tubules. Interspersed among the myofibrils, there are mitochondria and inclusions of glycogen. Most organelles are found between the myofibril bundles and the plasma membrane.
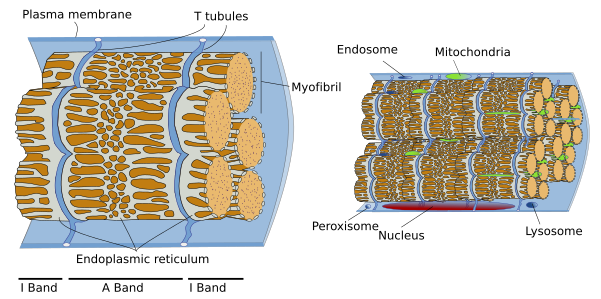
When SSMC are observed at light microscopy in a longitudinal view, a pattern of dark and light striations is visualized (Figures 1, 2, and 4). That is why they are called striated. Striations are perpendicularly oriented to the long cellular axis. This striated pattern is a consequence of the organization of the cytoskeleton filaments that form the myofibrils. Dark striations are called A bands (A stands for anisotropy, which can be observed with the polarization microscope) and light striations are known as I bands (I stands for isotropy). A division line can be observed inside each A and I bands, known as Z and H discs, respectively. Z discs has a zigzag organization when observed at electron microscopy. H discs have an additional inner darker line called M line. The portion of myofibril between two consecutive Z discs are known as sarcomere.
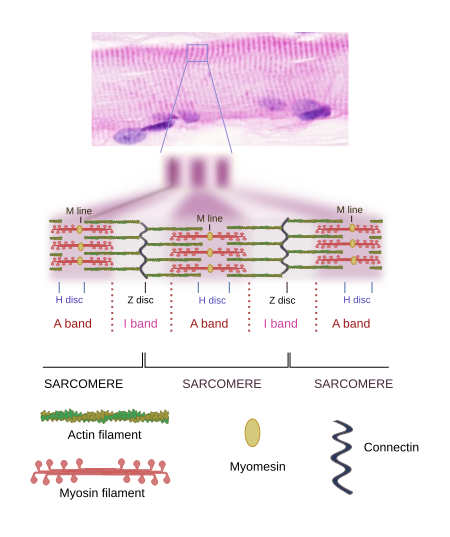
2. Sarcomere organization
The organization of SSMC sarcomeres is the result of the arrangement of a wide variety of proteins (Figures 4 and 5). Let's see some of them and their functions.
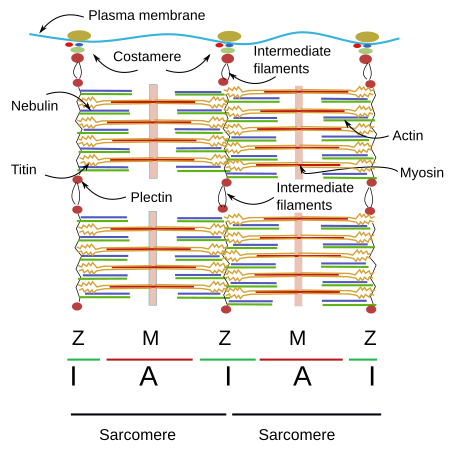
Thin filaments
Actin. Actin molecules are the main component of thin filaments in the sarcomere. Each thin filament is composed of two coiled alpha-helix of actin proteins associated to tropomyosins and troponins (Figure 6). Mature thin filaments are 1 µm long, and this length is extraordinarily constant in SSMCs thanks to the influence of many associated proteins (see below). Vertebrates express several actin isoforms showing slightly differences in their amino acid sequence that are expressed in different cell types and in particular developmental stages. Two of them are specifically expressed in the SSMC and cardiac muscle, and two can be expressed in SSMCs and in other tissues.
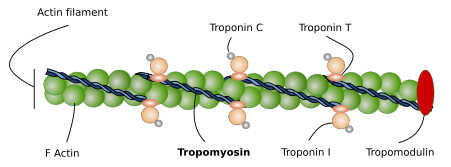
Tropomyosin. It consists of two amino acid helical alpha-chains, which are associated to actin filaments. There are several tropomyosin isoforms derived from alternative splicing of 4 genes. Tropomyosin chains are as long as the actin filaments and are placed at the grooves left by the actin proteins. The main role of tropomyosin, together with troponin, is to regulate the interactions between actin filaments and myosin II heads. Tropomyosin may change its spatial conformation, and may allow or inhibit the physical contact between actin filaments and myosin II heads. During muscle relax, tropomyosin does not permit the actin-myosin contact. The increase in calcium concentration after the electrical stimuli makes possible the actin-myosin interaction, and therefore the effective muscle contraction. It is interesting that the precise position of tropomyosin over the actin filament may change slightly depending on the tropomyosin isoform expressed.
Troponin. There are three types of troponin associated to actin filaments: C, I and T. The three molecules form a complex that cooperates with tropomyosin to regulate actin filament and myosin filament interactions. There are several isoforms of troponin types, so that cell expression of different isoform regulates the muscle cell contraction in a slightly different way. Troponin mechanism in muscle cells is not fully known, but 25 % of hypertrophic cardiomyopathies are caused by mutations in troponin genes.
CapZ and tropomodulin. These are proteins attached to the ends of actin filaments that control the length and shortening of actin filaments. CapZ joins the plus end and influences the nucleation and stability of actin filament. CapZ is a heterodimer formed of alpha and beta units found in the Z line, where actin filament is attached to. Tropomodulin is linked to the minus end, the free end of the actin filament. Its ability to join and stabilize the minus end depends on the presence of tropomyosin molecules. Tropomodulin is essential to establish the total length and stability of the actin filaments, which determines the contractile properties of the muscle cell. For example, tropomodulin defects may cause longer actin filaments, leading to slower contraction rhythms.
Thick filaments
Myosin. Thick filaments of the sarcomere are made up of myosin II and several associated proteins (Figure 7). There are hundreds of myosin II proteins in each thick filament. Myosin molecule consists of a globular domain, or head, that generates the movement, and a lineal domain linked to other lineal domains of other myosins molecules. All the lineal domains together form the stem of the thick filament. There are several myosin II isoforms that may affect the activity of the globular domain and are expressed selectively in different types of muscles. The globular domains of the myosin molecules interact with the actin thin filaments to produce movement. Other proteins associated with the thick filament are titin, C and H proteins, found in the C line, and AMP-desaminase.
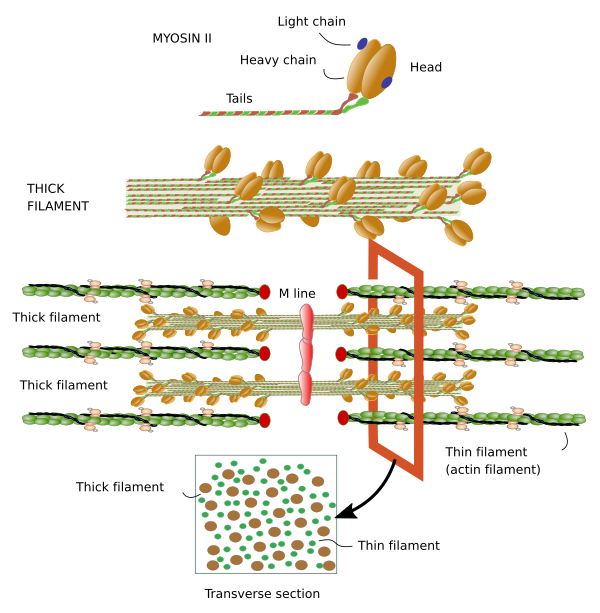
M line is the area where the thick filaments are anchored. Myomesin is a protein found in M line that binds myosin and titin, and might develop a relevant structural role. In cardiomyocytes and fast contraction SSMCs, M line contains the M protein. MURF-1 is another protein of M line that binds titin.
Other proteins
Titin can be regarded as the third filament of the sarcomere. After actin and myosin, titin is the third most abundant protein in muscles and is the longest protein found so far (38138 amino acids). It is anchored to the Z line by the amino terminal end, and to the M line by the carboxyl terminal end. Both ends of the titin of one sarcomere overlap with titins of adjoining sarcomeres forming a continuous scaffold. The segment of the protein found in the I Band is elastic and may counteract the sarcomere stretching, as well as limiting the maximal length of the sarcomere.
Nebulin is another giant protein, extending from the Z line to the free end of the actin filament. It is a protein that cannot be stretched, and this property makes nebulin a good candidate to control the exact length of the actin filament. It also may modulate the interactions between actin and myosin filaments. Curiously, nebulin has not been found in cardiac muscle cells, although other similar proteins may perform its function in these cells.
There are many intermediate filaments associated to the Z line that probably help with the lateral connections of myofibrils. One of these intermediate filaments is desmin, which is also associated with costameres (see below). Desmin interlocks Z disc and establishes connections between Z line and the plasma membrane, nuclei, mitochondria, and probably microtubules. There are also proteins working as transducers of signals that may exchange their position between the Z line and other cell locations. Alpha-actinin is a protein linking actin filaments between one another, MLP helps to keep the sacomere integrity and connects myofibrils and nuclei. Other proteins are FATZ, myopaladins, filamins, telotonins, and many others.
Costameres
Myofibrils of SSMCs must be anchored to proteins of the plasma membrane. Costameres are protein complexes found in the plasma membrane that are anchoring points for Z and M lines. SSMCs are wrapped by a special extracellular matrix called basal lamina that provides structural integrity to the cell. Costameres are the intermediary structures that link myofibrils with the basal lamina. Integrins are among the proteins mediating this anchoring that, in addition, work as mechanical sensors of cell stretching. A central protein of costameres is dystrophin, which binds the laminins of the basal lamina. Dystrophin mutations cause muscle dystrophies. Spectrin is another protein found in the costameres that collaborates in the connection between costameres and the cytoskeleton.
Intermediate filaments are cytoskeleton components that cooperate with miofibrills and plasma membrane proteins to maintain the cellular integrity. Plectin is an intermediary key protein between miofibrils and intermediate filaments. Ankyrins are proteins that facilitates the binding of costameres to the plasma membrane, and they also help to organize other plasma proteins, such as ion channels and pumps, in specific membrane domains.
Some proteins associated to the sarcomere have been found within the nucleus too. These proteins can cross the nuclear envelope and are thought to be messengers carrying information to the nucleus about the state of the sarcomeres.
Contraction mechanism
The function of SSMCs is contraction and therefore the movement of animals. This cellular shortening is done by the activity of the cytoskeleton. The contraction unit is called sarcomere, which is the segment of the myofibril between two consecutive Z discs. This is about 4 µm in length in a relaxed muscle. During contraction, Z discs get as close as 1 µm. This happens because I bands are shortened when actin filaments are dragged over the myosin II filaments. The A band keeps the same length.
Shortening of I band can be explained as follows. Thick filaments are organized in a way that the myosin II molecules of one half of the filament show antiparallel orientation when compared with the myosin II molecules of the other half (see Figure 7). So, the traction forces in the two halves are toward the center of the thick filament, and each half is dragging actin filaments from different I bands.
3. Innervation
SSMCs are innervated by axons of motoneurons, neurons that can be located in the brain or in the spinal cord. Each motoneuron is able to contact several SSMCs, but a SSMC receives innervation from only one motoneuron. A motor unit is a motoneuron and all SSMCs it innervates. Motoneuron axons make special synaptic contacts onto SSMCs known as motor plates or neuromuscular junctions, formed by an axon terminal, SSMC plasma membrane and Schwann glia as a warpeer. The neurotransmitter released in motor plates is the acetylcholine. Some SSMCs are also innervated by sensory terminals forming the encapsulated receptors and tendinous receptors, both carrying information about the contraction and position of SSMCs.
4. Types
Histochemical techniques are able to distinguish 3 types of SSCM: red, intermediate, and white. The three types are present in each muscle, but with different proportions according to the type of muscle. They differ in the amount of myoglobin, which is a protein of SSMCs that transports oxygen. Red SSMCs are short cells with a high concentration of myoglobin and many mitochondria, that allow a slow and sustained contraction. They are abundant in those muscle responsible for keeping the body posture. White SSMCs contain a lower amount of myoglobin and fewer mitochondria, and are longer cells. They are able to make fast contraction, but they fatigue quickly, and participate in fast and precise movements. Intermediate fibers show properties of both types.
-
Bibliography ↷
-
Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. 2002. Striated muscle cytoarchitecture: an intricate web of form and function. Annual review of cell and development biology. 18: 637-706.
-