Enterocytes are columnar cells that form most of the epithelium of the gut intestine (Figures 1 and 2). They are more abundant in the small intestine than in the large intestine and appendix. In the small intestine, the amount of enterocytes is about 80 % of the total epithelial cells. The main function of enterocytes is to absorb molecules from the gut lumen and their transport toward the surrounding connective tissue and blood vessels. It is of notice that the gut epithelium is the larger surface of the body in contact with the external environment (the lumen of the gut is the external environment), larger than the skin.


1. Morphology
Enterocytes have microvilli in their apical (free) surface (Figure 3), many mitochondria in the basal part, and well-developed Golgi apparatus and endoplasmic reticulum. The mechanical integrity of the intestine epithelium, that is, the cohesion between enterocytes and the lack of intercellular passages, depends on the cell junctions between adjacent enterocytes (Figure 4). Tight junctions and adherent junctions are found close to the apical domain of the enterocyte. Desmosomes are located in the latero-basal membranes. Gap junctions are observed in the latero-basal membranes as well. Hemidesmosomes are found in the basal plasma membrane of enterocytes attaching the cell to the basal lamina.
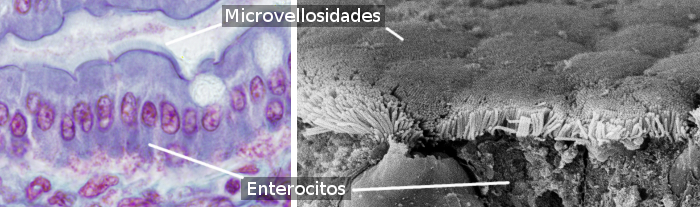

Enterocytes have two domains: apical and baso-lateral (Figure 4). That is why it is said they are polarized cells. Polarization is produced by the activity of a well-arranged cytoskeleton and well-developed vesicular traffic that distribute molecules differentially between both domains. Tight junctions prevent lateral diffusion of molecules between both domains, thus helping to maintain the polarity. Enterocytes show a highly packaged microvilli in the apical domain, that increases the membrane surface about 100 times (Figure 3). In the apical membranes, there area many transporters that work as gates for molecules resulting from digestion to come into the enterocyte. There are other transporters in the baso-lateral membranes for these molecules to exit the enterocyte and reach the blood vessels. This segregated distribution of receptors is generated by vesicular trafficking.
2. Life cycle
The intestine lumen is full of potentially toxic molecules that harm the enterocytes. Instead of repairing every insult, damaged and older enterocytes die by apoptosis and extruded from the epithelial layer, and continuously replaced by new ones. Small intestine mucosa is highly folded and forms many evaginations, or villi, and invaginations known as Lieberkühn crypts. In the large intestine, there are Lieberkühn crypts, but no villi, and the epithelial surface is flat. The life cycle of the enterocyte begins at the deepest part of the Lieberkühn glands and ends at the tips of the villi of the small intestine or in the epithelial surface of the large intestine. The life of an enterocyte is about 2 to 5 days long. In humans, the intestinal epithelium is renewed every 4 to 5 days.
Enterocytes develop from adult stem cells that are found at the bottom of the Lieberkühn crypts (Figure 5). Initially, adult stem cells become transient amplifying cells (progenitor cells), which are located a bit further from the adult stem cell niche. Transient amplifying cells divide 4 to 6 times to increase the progenitor population and then differentiate into the variety of cell types found in the intestinal epithelium. Most of them become enterocytes, but also globet cells, M cells, and the other cell types. New enterocytes are pushed progressively toward the tips of the villi or to the epithelial surface by new cells. Once they reach these positions, they die and are extruded from the epithelium. Extrusion involves both mechanical pressure and lost of adhesion connections with neighbor cells. Some cells die by apoptosis and then are expelled from the epithelium. It is not known what is the mechanism for the movement of enterocytes from the crypts toward the epithelial surface. Molecular components of the basal lamina are different along the enterocyte path, and it is thought that it can contribute to this migration. The diet has also been thought to be involved in the dynamic of the life of enterocytes.

3. Functions
Digestion
The main function of enterocites is to absorb nutrients after the stomach and enzymatic digestion of food. Enterocytes may use glutamate and glutamine, as well as fatty acids and glucose, as their energy supply. This is curious because all types of nutrients go through enterocytes. Enterocytes also help with digestion by secreting enzymes that degrade peptides and disaccharides. The glycocalyx of the enterocyte apical domain forms a layer of about 400 to 500 nm thick, sometimes even 1 µm thick. Some enzymes that participate in digestion are anchored to this glycocalyx. Thus, enterocytes not only select and catch substances from digestion, but they also process some of them. Actually, it is said that there are two phases in the digestion, one happening in the lumen of the intestine, carried out by pancreatic enzymes, and the other one in the surface of the enterocytes, performed by other digestive enzymes. Most of the nutrient absorption is accomplished by the small intestine enterocytes, whereas enterocytes of the large intestine mainly absorb water. In addition, small vesicles are released from the tips of the microvilli containing enzymes like phosphatases, which may have a defense function against pathogens.
SSubstances resulting from digestion have to cross the intestinal epithelium to reach the blood stream. It can be done through several pathways: transcellular, endocytosis/transcytosis and paracellular.
Transcelullar.Most molecules cross the epithelial layer of the intestine by crossing the enterocytes. First, they cross the apical membrane and then the basolateral one. Molecules are moved by free passive diffusion, facilitated passive diffusion, or active transport. In the free passive diffusion, molecules cross membranes without any help. In the facilitated and in the active transport, molecules need to be recognized by specific transporters inserted in the membranes. Water, ethanol and many lipids cross through enterocytes by free passive diffusion. Glucose, some lipids, amino acids, and di- and tri-peptides enter enterocytes by facilitated passive transport or by active transport.
T he apical domain of enterocytes bears a set of proteins for absorption of substances, whereas the latero-basal membranes have another transmembrane transporters for getting molecules out of the cell.
The absorption capability depends on differentiation stage of the enterocyte, which determines the number of sodium membrane pumps, more abundant as enterocytes move further away from the deep of the crypts. Thus, most absorption of sugars and amino acids is done in the upper third of the villi of the small intestine and near the surface of the large intestine. For example, hydrolase activity increases as enterocytes move away from the stem cell niches (deep parts of the crypts).
The absorption of glucose is a typical example of absorption mechanism. Glucose crosses the apical membrane of the enterocyte by co-transport coupled to a sodium gradient. This sodium gradient is generated by sodium/potassium pumps, and allows glucose to enter the enterocyte against the glucose concentration gradient. The sodium glucose transporter (SGLT) is responsible for this co-transport. On the other hand, the GLUT2 transporter is found in the laterobasal membranes, which translocates glucose from the cytoplasm to the intercellular space. GLUT2 is able to transport glucose in both directions: inward and outward of the cell. However, as glucose concentration is higher within the cell, the net transport of glucose is outward. Thus, SGLT increases glucose concentration in the enterocyte cytoplasm and GLUT2 allows glucose to escape toward the blood vessels. The precise location of the two transporters produces a flux of glucose through enterocytes, from the intestine lumen to the blood vessels.
Fat is among the most energetic substances, and it is necessary for cellular membranes. The majority of the fat we eat is incorporated from the intestine in triacylglycerol form, although other types can be absorbed too, such as cholesterol. First, pancreatic lipases degrade meal fat in the intestine lumen and triacylglycerols are converted to fatty acids and monoacylglycerols (Figure 6). These molecules, together with cholesterol, fat soluble vitamins, and phospholipids, form micelles, which are small lipid droplets soluble in water thanks to the biliary acids. Micelles cross freely the enterocyte apical membrane. CD36 y FABP (fatty acid binding protein) transporters make possible for some fatty substances to cross the enterocyte apical membrane by facilitated passive transport. The bulk of the fat transport is as micelles, whereas the membrane transporters look like a sensory system to detect fatty acids with long chains in the intestine. Cholesterol, as an individual molecule, can be also transported by NPC1L1 (Niemann-Pick C1-like 1) transporter, which transfers cholesterol from the intestine lumen to the enterocyte cytoplasm.

Once in the enterocyte, fats are joined to some proteins and moved to the endoplasmic reticulum, where triacylglycerols are synthesized again. Then, these lipids are combined with some proteins to form lipoproteins called pre-chylomicrons. ApoB protein is synthesized in the endoplasmic reticulum. ApoB, together with MTP (microsome tranfer protein) and the fatty acids form this lipoprotein primordial particle. In the smooth endoplasmic reticulum, ApoB is substituted by Apo A-IV protein. Fat absortion boost the production of A-IV apopliprotein, which locate at the surface of chylomicrons. A-IV inhibits the hungry sensation. All these components constitute the pre-chylomicrons in the smooth endoplasmic reticulum, are included in vesicles and moved to the Golgi apparatus. Here, pre-chylomicrons are joined together to form chylomicrons, which are included in exocytic vesicles and released at the laterobasal domain of the enterocyte. In this way, chylomicrons can reach blood and lymphatic vessels. Chylomicrons are lipoproteins with a body mainly compose of triacylglycerols and a coat of phospholipds, cholesterol and apolipoproteins. They play a major role transporting triacylglycerols and liposoluble vitamins. Besides chylomicrons, fat associate to form very low density lipoproteins (VLDLP), which are also exocyted, or can be temporary stored within the enterocytes as lipid droplets.
Once exocyted from the enterocyte, chylomicrons enter lymphatic vessels of the intestinal villi, and then in the lymphatic myenteric plexuses from which they pass to the blood vessels.
Enterocytes also uptake the iron after digestion. Iron is important for many proteins, such as hemoglobin. It can be found in food as part of hemo groups or bound to ferritin (in animal meat) (Figure 7). After digestion, free iron enters enterocytes through DMT1 transporter (divalent metal transporter 1). Transgenic mice lacking this transporter develop severe anemia. DMT1 is a co-transporter coupled to a proton gradient. The proton gradient is generated by Na+/K+ pump, also found in the apical membrane of enterocytes. DMT1 transport Fe2+ but most irons from meal is in the Fe3+ form. Iron reduction (Fe3+ to Fe2+) is carried out by a reductase enzyme located in the apical surface of the enterocyte. On the other hand, the iron bound to hemo groups appears to be incorporated by receptor mediated endocytosis. Once within the enterocyte, the hemo group is degraded and the iron can get into the cytosol.

Whatever the entry pathway, once in the cytosol, the movement of iron toward the basolateral membranes appears to be mediated by metallochaperone proteins. In the basolateral membranes, iron is translocated to the extracellular space by the ferroportin transporter. Ferroportin transports the Fe2+ form. However, ferritin is the protein that transports iron from the extracellular space to the portal vein system, and iron must be in the Fe3+ form. There are ferro-oxidases in the basolateral membranes of the enterocyte that make possible the conversion from Fe2+ to Fe3+. Iron can be stored in the cytosol of the enterocyte bound to ferritin.
Endocytosis/transcytosis. Some molecules, such as immunoglobulins, enter enterocytes by receptor mediated endocytosis and transported to basolateral membrane domains by transcytosis. The journey begins in vesicles formed at the base of the microvilli, and that are fused later with endosomes. Endosomes release new vesicles that fuse with the basolateral membranes by exocytosis. In this way, molecules escape the lysosomal degradation pathway.
Paracellular. Water and ions cross the epithelium by paracellular pathway.
Protection
Enterocytes have to form a barrier that reject antigens, toxic molecules and microorganisms, but at the same time let cross nutritive substances. Enterocytes are in contact with many microorganisms. Those microorganisms that are resident in the intestine are usually beneficial, but can be dangerous if they reach the internal tissues, and there are also those non-resident pathogens that come with the meal. The apical surface of the enterocytes is covered with a layer of mucous substances released by globet cells. This layer is composed of carbohydrates and has a dense viscosity that allows the diffusion of molecules, but rejects cells and the largest molecules. In addition, enterocyte microvilli show a well-developed glycocalyx in the apical tips of each microvillus, which acts as a physical and electrical barrier since it is full of negative charges. These microvilli make difficult a direct physical contact between microorganisms and enterocyte membrane. However, even if they pass these two barriers, microorganisms should overcome the inner transport mechanism of the microvilli.
Mucins are highly glycosylated proteins found in the apical plasma membrane of enterocytes. They contribute to the well-developed glycocalyx. Mucines are transmembrane proteins linked to the cytoskeleton by their cytosolic domain. MUC3, MUC12 and MUC17 are the most abundant mucins. They contain about 5000 amino acids and the carbohydrate component can extend up to 1 µm from the cell surface. Mucins form a physical barrier hard to cross by bacteria.
Enterocytes are able to start and regulate inflammatory processes by releasing several chemokines and cytokines. They also have receptors for these molecules. Thus, enterocytes release pro-inflammatory molecules that influence immune cells found in the intestinal mucosa.
Another less known protection mechanism is the release of vesicles from the apical surface of enterocytes. Actin and myosin, the motor apparatus of microvilli, develop mechanical forces that drag membranes toward the tip of each microvillus. The membrane accumulation ends up as vesicles, which are released into the intestinal lumen. These vesicles contain a high amount of alkaline phosphatase that is a powerful as anti-pathogen agent by reducing lipopolysacharide toxicity and intestinal inflammation. It also hinders the attachment of bacteria to the intestinal epithelium and decreases the proliferation of bacteria. The released of vesicles by enterocyte are a way of sending anti-microbe molecules to regions far away from the epithelium.
-
Bibliography ↷
-
Bibliography
Barker N. 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature. 15: 19-33.
Giammanco A, Cefalù AB, Noto D, Averna MR. 2015. The pathophysiology of intestinal lipoprotein production. Frontiers in physiology. 6: 61.
Knutson MD. 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature review in molecular cell biology. 15:19-33.
Knutson MD. 2017. Iron transport proteins: gateways of cellular and systemic iron homeostasis. Journal of biological chemistry Nature review in molecular cell biology. 292: 12735-12743.
Shifrin DA, McConell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ. 2012. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Current biology. 22: 627-631.
Snoeck V, Goddeeries B, Cox E. 2005. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes and infection. 7: 997-1004.
-