1. Structure-composition
2. Acid hydrolases
3. Functions
- Degradation
- Metabolic sensor
- Exocytosis
4. Orgánulos relacionados
5. Patologías
In the late 19th century, Metchikoff and coworkers proposed that phagocytosed material is digested in acidic intracellular compartments. These compartments were described by C de Duve (1955). He named them lysosomes. They have been found in all eukaryotic cells so far. Contrary to endosomes, lysosomes do not contain receptors for mannose-6-phosphate and show a lower pH. Their main function is degrading molecules coming from endocytosis (external environment) and autophagia (internal environment). Currently, they are regarded as key players in sensing the metabolic state of the cell and cell membrane repairing.
1. Morphology
Lysosomes are organelles of variable size, from 100 to 150 nm in diameter, with a limiting membrane (Figure 1). Depending on the physiological activity of the cell, the lysosomal population may account for 5 % of the total cellular volume. The lysosomal pH is about 5, which is optimal for the acid hydrolases activity (that is why the name "acid"). This low pH is achieved by proton pumps (v-ATPase: vacuolar proton pump) that fetch protons from the cytosol and get them into the lumen of the lysosome. The membrane of lysosomes protect the rest of the cytoplasm from this danger acid environment. The luminal surface of lysosomal membrane is coated with a dense layer of carbohydrates of about 8 nm in thickness, which is known as "lysosomal glycocalyx". These carbohydrates are part of glycoproteins and glycolipids of the membrane and form a physical barrier that prevents acid hydrolases to be in contact with and degrade de membrane. However, even if lysosomal membrane breaks, the cytosolic pH, about 7.2, would be basic enough to inhibit the activity of acid hydrolases.

There are different types of lysosomes regarding morphology and molecular content. In addition, they can be found in different positions in the cytoplasm. For instance, perinuclear lysosomes show a lower pH than peripheral ones. Peripheral lysosomes are involved in plasma membrane repairing and regulate the nutrient availability by the associtation with mTORC1. During starvation, peripheral lysosomes are targeted to perinuclear regions to perform autophagia. Hence, autophasomes are also moved to the perinuclear positions to get fused with lysosomes.
According to the degradation state of the luminal material, lysosomes are referred to as primary, secondary and residual bodies. Residual bodies contain material that can not be further degraded, and they are stored in the cell or fused with the plasma membrane for releasing the content into the extracellular space. Any flaw of a lysosomal degradative enzyme may lead to serious cellular malfunction because the product that should be degraded are stored intracellularly as residual material. For instance, the lack of beta-glycosylase lysosomal enzyme leads to the glycogenosis type II disease. This enzyme catalyzes glycogen, and when it is not present, glycogen depots are formed in the organs, which are usually lethal for the organism.
Lysosomes bear hundreds of integral and associated proteins, including transporters, pumps and ionic channels. Transporters translocate the end-products of the degradative process from the lumen to the cytosol, such as amino acids, carbohydrates and nucleotides. Sodium, potassium and chlorine cross the lysosomal membrane through ion channels. The flux of ions across the lysosomal membrane generates a membrane potential of about -40 to -20 mV. This membrane potential influences the proton gradient and other lysosomal activities. Sodium is the more abundant ion in the lysosomes.
Genes for lysosomal proteins, from acid hydrolases to membrane transporters, contain a DNA sequence in their promotor called CLEAR. This sequence is the target for TFEB and MITF transcription factors. TFEB is one of the molecules that increases the number of lysosomes and also boosts autophagy, so that both mechanisms are co-activated. During cellular homeostasis, TFEB is found in the cytoplasm. However, TFEB gets into the nucleus when the food supply is reduced, and therefore increases lysosomal activity and proliferation.
Early endosomes has to maturate to become late endosomes, and then gets fused with lysosomes for molecular degradation. However, the destiny of some molecules of the early endosomes is not degradation. To avoid lysosomes, these molecules are sent back from early endosomes to the plasma membrane or to the Golgi apparatus. Most molecules in the late endosomes end up into lysosomes by fusion the two organelles. Sometimes, the fusion is not complete and a bridge of communication is transiently established, through which molecules are transferred to the lysosome. Then the bridge is closed, and the two organelle remain as independent compartments.
Lysosomes have to be regenerated after a degradation process to keep up functional for another round of degradation. For instance, during the intense autophagy that happens in starvation, the number of functional lysosomes greatly decreases after 4 hours, but it is recovered again after 11 hours. It has been observed that new lysosomes are formed from small membranous tubules extended from autophagolysosomes. The cytoskeleton and the motor protein kinesin are involved in this process of tubulation. At the tips of the tubules, small vesicles are released mediated by the phosphoinositide PIP(4,5)P2 and the adaptors AP2 and AP4, which recruit clathrin. Once the small vesicles are free, they grow and get progressively acid to become mature lysosomes. The initial vesicles are called protolysosomes because they mature into functional lysosomes, and the process is known as ALR (autophagic lysosome reformation). A similar process happens after the fusion of phagosomes, coming from phagocytosis, with lysosomes.
The number of lysosomes per cell is driven by cell needs. For instance, during starvation, cells increases the number of lysosomes for degrading dispensable intracellular content and then get energy. During cell division, cells produces new lysosomes to be shared out by the two daughter cells. Getting new lysosomes, or increasing their size, are coordinated processes involving protein synthesis and vesicular trafficking.
Ggenes for lysosomal and autophagy proteins need to be expressed if the cell needs to increase the number of lysosomes. TFEB and TFE3 are transcription factors that promote the expression of these genes. Phosphorylation and dephosphorilation of the TFEB and TFE3 determine the crossing of the nuclear envelope to access the chromatin. Dephosphorylation label them to get into the nucleus and starts gene expression.
2. Acid hydrolases
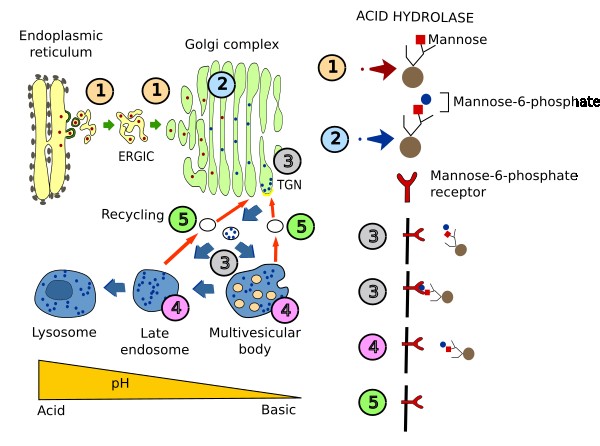
Acid hydrolases have to be targeted to lysosomes because they are the degradation machines. About 60 different lysosomal acid hydrolases have been found for degrading proteins (proteases), lipids (lipases), carbohydrates (glycosylases) and nucleotides (nucleases). These enzymes are synthesized in the endoplasmic reticulum. In the TGN of the Golgi apparatus, they are included in vesicles and are shipped to the multivesicular bodies / late endosomes. How acid hydrolases are selected in the TGN is described in the Figure 2. Briefly, in the Golgi apparatus, a phosphate group is added to a mannose of the enzyme. Mannose-6-phosphate is recognized by a membrane receptor in the TGN, and the cytosolic domain of the receptor interacts with adaptor proteins, which in turn interact with a clathrin coat. Thus, receptor-hydrolase complexes are gathered and included in vesicles targeted to multivesicular bodies/late endosomes, and end up later in lysosomes. There are other proteins needed by lysosomes that do not follow this selection process. For example, some transmembrane proteins, such as proton pumps, contain an amino acid sequence in their cytosolic domain which is recognized by adaptor proteins. Acid hydrolases are necessary for normal functioning of cells. Any mutation in any of these enzymes may cause the accumulation of non-degraded molecules that can end up in several pathologies. For example, non-degraded lipids may cause collateral damages in mitochondria and may inhibit autophagy.
3. Functions
Catabolizing molecules is essential for the cell. Acid hydrolases degrade in lysosomes molecules coming from extracellular and intracellular sources. This activity is not always intended to get energy and molecular building blocks. Lysosomes function also as nutrient and energy sensors, remove non-functional organelles or cell components, finish the mitotic signals, remove intracellular pathogens, release content into the extracellular space, participate in tissue and cell remodeling, and so for.
Degradation
There are three pathways to lysosomes for molecules to be degraded (Figure 3):

a) Lysosomes are the last station for the degradation endocytic pathway. Molecules in this pathway must reach lysosomes via endosomes. Those molecules of the endosomal compartment not recycled to the plasma membrane, or sent back to the TGN of the Golgi apparatus, are targeted to lysosomes through a default pathway.
Transmembrane proteins of the plasma membrane are targeted to lysosomes after the ubiquitination of their cytosolic domain. Ubiquitination is the addition of ubiquitin proteins. The ubiquitinated proteins are recognized by the sorting machinery of early endosomes, which does not gather these integral proteins for being included in vesicles shipped to plasma membrane. Ubiquitinated transmembrane proteins are concentrated at endosomal domains, where clathrin coats are present. So, ubiquitinated proteins are retained in the early endosomes, later found in multivesicular bodies / late endosomes, and finally in lysosomes, where they are degraded. This mechanism is used for degradation of membrane receptors, adhesion molecules, transporters and channels. Non-ubiquitinated integral proteins at early endosomes are usually included in vesicles back to the plasma membrane.
b) Particles brought into the cell by phagocytosis follow a distinct intracellular pathway. Bacteria, viruses and cellular fragments are engulfed by phagocytosis and form a compartment that develops and becomes a phagosome. The content degradation takes place when the phagosome fuses with lysosomes.
c) A third pathway for molecules to get to lysosomes is autophagy. It is a cellular ubiquitous process for degrading cytoplasmic material, organelles and cytosolic molecules. Lysosomes are involved in the different types of autophagy. For example, in macroautophagy, cytoplasmic material is enclosed by inner membranes and this new membrane bound compartment, known as macroautophagosome, fuses with lysosomes and the content is broke down.
Metabolic sensor
Lysosomes are thought to influence the metabolic state of the cell. Two populations of lysosomes have been identified with different distribution in the cytoplasm. A perinuclear population of lysosomes is involved in molecular degradation, whereas a peripheral population is more related to sense resources availability for the cell. These peripheral lysosomes are also involved in membrane repairing after plasma membrane breakages.
mTOR (target of rapamycin) is a serine/threonine kinase well conserved during evolution. There are two isoforms: mTORC1 and mTORC2. mTORC1 influences cell grow and division, and responds to low nutrient levels, growth signals, and the energy level of the cell, by balancing anabolism (synthesis) and catabolism (degradation). mTORC1 is found at the surface of lysosomes, and this location is very important to perform its function. At the lysosomal membrane, there are molecules that can sense the amount and differences of amino acids between inside and outside the lysosome. mTORC1 is activated when there are nutrients enough. When nutrients are low, mTORC1 is inactivated and macroautophagy is triggered. mTORC1 inactivation stimulates the expression of genes that start macroautophagy and the molecular machinery that moves lysosomes from the periphery to the perinuclear region of the cell. This new location of lysosomes facilitates the formation of autophagolysosomes (lysosomes and autophagosomes), which are compartments for degradation of macroautophagy material. This material is compose of inner cellular content (cytosol and organelles), and becomes a source of energy for the cell.
Exocytosis
It has been long thought that lysosomes have very little connection with vesicular trafficking, and they were regarded as a terminal compartment. However, many evidences have being reported about lysosomes involved in regulated exocytosis processes. For example, in the liver, several lysosomal enzymes are released and are part of bile. Melanocytes may release melanosomes (granules with melanin that are taken by keratynocytes and give a dark color to the skin), which share similar features with lysosomes. The sperm achrosome is a vesicle full of hydrolytic enzymes, which is released during fertilization. It has also been proposed that cells may get rid of waste products (molecules that cannot be longer degraded) by lysosomal fusion with the plasma membrane. Lysosmes are also involved in repairing large breakages of the plasma membrane by helping with their own membrane as a patch (see Membrane repairing).
4. Lysosomal related organelles (LRO)
Some cells contain organelles that can be related to lysosomes because of their molecular content and physiological features, or because they derive from lysosomes. These organelles are known as LRO (lysosomal related organelles), and include melanosomes of melanocytes, lytic granules of T lymphocytes, dense granules of megakaryocytes, lamellar bodies of the lung type II cells, Weibel-Palade bodies from endothelial cells, and granule of osteoclasts. LRO release their content after the cell sense some stimuli.
Yeast do not show typical lysosomal, but structures known as vacuoles. Vacuoles and lysosomes share many features, such as acid hydrolases, membrane transporters and proton pumps. They can store a large amount of amino acids, which has impact on yeast physiology because it allows to overcome starvation periods. The amount of amino acids in the vacuoles is also increased by autophagy.
5. Pathology
More than 60 diseases are linked to lysosome flaws. Malfunction of acid hydrolases or membrane transporters lead to severe pathologies referred to as lysosomal store disorders. They include metabolism disorders, neurodegeneration, and growth inhibition. For instance, the type C Niemann-Pick disease is caused by the lack of the cholesterol transporters NPCI and NPCII, leading to the amassing of cholesterol within lysosomes. The ameliorated activity of lysosomes that happens during aging contributes to the general problems of aging process.
-
Bibliography ↷
-
Bibliography
Delevoye C, Marks MS, Raposo G. 2019. Lysosome-related organelles as functional adaptations of the endolysosomal system. Current opinion in cell biology. 59: 147-158.
Perera RM, Zoncu Z. 2016. The lysosome as a regulatory hub. Annual review of cell and developemental biology. 32: 223-253. doi: 10.1146/annurev-cellbio-111315-125125.
Yang C, Wang X. 2021. Lysosome biogenesis: regulation and function. Journal of cell biology. 220: e2021020001. https://doi.org/10.1083/jcb.202102001
-
 Endosomes
Endosomes 