1. Mechanisms
- Caspases
- Receptors
- Stress
- Pathogens
2. Cell level
3. Development
- Morphogenesis
- Function adjustment
4. Homeostasis
Apoptosis is a molecular mechanism of eukaryote cells that ends up with cell death. It is a cellular suicide performed by an autodestruction molecular program triggered either by extracellular or intracellular stimuli. Apoptosis is also known as programmed cell death because the cell takes a precise and ordered chain of steps. It does not mean that cells are all going to die by apoptosis, that is, there is no apoptosis if there is no apoptotic stimuli.
In pluricellular organisms, apotosis is involved in many physiological and pathological processes. For example, morphogenesis during embryo development, tissular homeostasis and regeneration in adults, withstanding pathogens and cellular stress, and cancer. The amount of cells that die by apoptosis is huge, both during development and in adults. Blood and epithelial renewing in adults is intense, which means that an enormous amount of cells die by apoptosis.
1. Molecular mechanism
The molecular mechanism for apoptosis has been conserved during evolution in most eukaryote cells. It is an ordered process and energy dependent, that needs to be initiated. A number of signals have been found to start apoptosis: extracellular signals that activate the so-called dead receptors, intracellular signals that activate organelles like mitochondria, and a third pathway that involves molecules like perforin and granzyme. These three pathways converge in degradative enzymes known as capasases (Figure 1.)
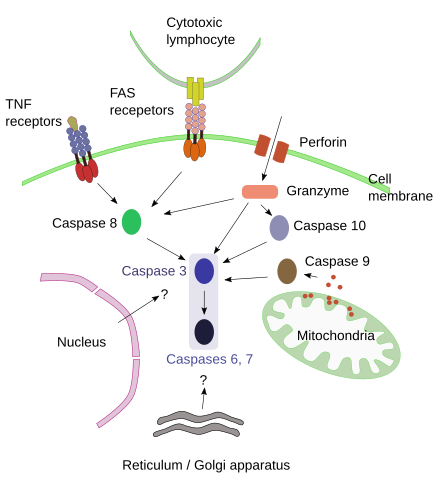
Figure 1. Main pathways for triggering apoptosis. Question marks inicate feasible mechanisms.
Caspases
Caspases are proteolytic enzymes synthesized and released into the cytosol as procaspases, which are inactive. Caspases are responsible for degrading components that ends up with cell death. There are several types of caspases, each of them specialized in degrading different proteins. All caspases break amino acid chains at a point where aspartate is found, but different caspases degrade different proteins according to the amino acids found close to aspartate. The first caspases to be active are 2, 8, 9 and 10 (initiator caspases), and then caspases 3, 6 and 7 are activated later (executioner caspases). There are very specific caspases and others, like caspase 14, only activated during the embryonary period. Among the executioner caspases, caspase 3 is considered very important because activates CAD endonuclease, which degrades chromatin. It also has influence in cytoskeleton reorganization, that allows splitting the cell in several independent fragments.
Once initiator caspases have been activated, the suicide process appears a non-stop mechanism, although it is not always so (se below). It is a feed-forward mechanism where caspases activate other caspases. Finally, executioner caspases are activated and cell degradation takes place. Curiously, caspases perform non-apoptotic functions like spermatids separation, macrophages differentiation, epidermis cornification, erythropoiesis and eye lenses differentiation.
Dead receptors. Extracellular signals.
Extracellular signals can start apoptosis by activating transmembrane receptors known as death receptors. Death receptors are included in the TNF (tumor necrosis factors) receptor family. Each receptor is activated by a specific signal or ligand. For example, ligand/receptor: FasL/FasR, TNF-α/TNFR1, Apo3L/DR3, Apo2L/DR4, o Apo2L/DR5. Ligand recognition make receptors to form groups and this association recruits adaptor proteins to receptor cytosolic domains. Adaptor proteins recruit procaspase-8. Altogether form a molecular environment that produces conformational changes in procaspases. These changes lead to autoproteolysis of procaspases, which make procaspases become active caspases. Caspases leave the membrane and start the degradative process in the cytoplasm. Apoptosis is started.
Cellular stress. Internal signals.
This pathway involves stimuli that are not directly mediated by receptors. For example, lack of survival factors, high radiation, high temperature, toxic substances, and other insults triggers apoptosis by increasing cellular stress. These types of stimuli modify the cellular physiology that ends up affecting the permeability of the inner mitochondrial membrane, and some proapoptotic molecules are released from the mitochondrial matrix to the cytosol. Cytochrome C is one of these proapoptotic molecules, which joins to apaf-1 and procaspase 9 forming altogether the so-called apoptosome. The molecular environment of the apoptosome produces the activation of procaspase 9 and the beginning of the apoptotic degradation process. Mitochondria also releases enzymes involved in later stages of apoptosis by entering the nucleus and digesting DNA. In the mitochondrial membrane there is a family of proteins known as bcl, which modulate, inhibit or boost apoptosis. They are potential targets to selectively fight against cancer cells.
Pathogens
Cytotoxic T lymphocytes are able to kill cells that contain pathogens by activating receptors that start apoptosis. They can also introduce molecules into the infected cell to activate apoptosis. Cytotoxic T lymphocytes contain granules with two molecules: perforin and granzyme. These granules are exocyted when infected cells are detected or when they recognize altered cells like cancer cells. Perforin is inserted in the plasma membrane of the infected cell and make pores, through which granzymes enter the cell. Granzymes (there are two types: A and B) activate caspases 10 and 3. They also stimulate mitochondria to start apoptosis as if they were an internal signal.
2. Cell process
During apoptosis, cells undergo several changes: cell retraction or shrinking, cytoplasm gets darker, organelles are more tightly packed, chromatin condenses, which is a change visible at light microscopy. Later, there are protrusions and folding of the plasma membrane so that the cell splits in several portions known as apoptotic bodies, which are always enclosed by membrane. Apoptotic bodies are phagocyted by macrophages. Since there is no release of intracellular molecules to the extracellular space through plasma membrane breakages, there is no inflammatory processes. In addition, macrophages do release any molecule after "eating" the apoptotic bodies. In this way, apoptosis is cell death without bothering the neighbor. However, opoptotic cells sometimes release molecules for cell proliferation, extracelular matrix remodeling and cytoskeleton reorganization.
Apoptotic bodies are quickly removed by macrophages, otherwise they can be broken, release their content and produce an inflammatory effect. Under macrophage activity inhibition, apoptosis leads to an inflammatory process. Macrophages recognize specific molecules on the external surface of the apoptotic bodies: phosphatidil serine, calreticulin and anexin I. During apoptosis, phosphatidil serine is transferred from the inner monolayer of the plasma membrane to the outer monolayer, so that macrophages are able to recognize a change in the lipid composition of the plasma membrane surface.
Once caspases have been activated, it has been accepted that apoptosis is irreversible. However, it has also been observed that after the inhibition of macrophages, some cells are able to recover after the activation of caspases. Thus, the role of macrophages would be to be sure that cells that start apopotsis are going to die for real.
Necrosis is cell death by natural causes like very high temperature, mechanical forces, and toxic substances. Necrosis is a passive and uncontrolled process that led to breakage of the plasma membrane and release of the cell content producing inflammatory processes. There is another type of cell death mediated by macroautophagy. Death by macroautophagy is m mechanism under the cell control.
3. Development
During the invertebrate C. elegans development, 1090 somatic cells are generated, and 131 of them die in very precise places and times. Over-exceeding cells that are later remove is observed in all animal species. It looks like a waste of energy, but looks are deceiving.
Morphogenesis
A good example of apoptosis shaping an organ is the elimination of interdigital membrane during embryo development. Fingers and toes are initially connected by sheets of cells that later die by apoptosis, and it results in the final shape of fingers and toes. This process is not working in the hind limbs of ducks and other aquatic birds. They use these interdigital membranes to get impulse in the water. Apoptosis of interdigital membranes is like sculpting a structure to get the final shape. Another example of apoptosis as a morphogenetic mechanism can be observed during metamorphosis of many species, particularly in amphibians. They reorganize the brain, the gut and get rid of the tail. The tail elimination is by apoptosis.
Death of some cell populations sets loose some mechanical tensions that facilitates the folding or shape modification of some embryo structures. For example, it appears to happen during the neurulation, where apoptotis may make the neuronal tube closure faster. The formation of the pro-amniotic cavity is the result of apoptosis in the middle of the embryo inner cell mass after the implantation of the embryo. This process is known as cavitation.
The final shape of body organs is the result of a balance between cell proliferation and cell death during development and adult stages. There are genes, like those of the Hippo pathway, involved in cell proliferation that inhibit apoptosis. When these genes are not active, apoptosis is favored and therefore the number of cells decreases.
Fine tune
For biological systems, it is cheaper to overproduce elements to get first a coarse structure and then selectively remove some of them to obtain a more functional and precise organ or structure. This is clear in the nervous system where to get a working brain from the beginning is far more complicated than establishing an initial gross basic wiring that is fine-tuned over the time by removing noisy or non-proper connections. Thus, those neurons that do not make very useful connection die. The same happens in the immune system. There is a massive production of lymphocytes by random reordenation of genes and those that recognized molecules of the body (they can produce autoimmune responses) are removed. In this way, about 60 genes are needed, but more than 100000 genes would be necessary for producing each line of lymphocytes. Overproduction and later removing non useful cells actually economizes resources and it is the mechanism of choice for morphogenesis and many cellular systems.
4. Homeostasis
In adult animals, apoptosis is a mechanism for balancing cell proliferation happening in many tissues. It is an ongoing process of cell death and replacing by new cells. Macrophages remove apoptotic cells. In most tissues, macrophages are up to 15 % of the total cell population. The balance between death and proliferation is clear in tissues like epithelia, where there is a continuous renewing of cells that keeps a nearly constant cell population. For example, epidermal keratinization is a special apoptotic process. Enterocytes show a life cycle that starts in the base of Lieberkühn crypts and ends in the intestine surface by apoptosis. Something similar occurs in the respiratory epithelium. The renewing mechanism is interesting for cell that are exposed to toxic or pathogenic agents because it is more efficient than invest in increasing resistance or improving repairing mechanisms. The balance between birth and death is also important for blood cells. Death/proliferation equilibrium are controlled in the bone marrow by hematopoietic stem cells, whose number is regulated by apoptosis, and therefore the number of mature circulating blood cells. Even the number of platelets are under apoptotic control, which is an example of apoptosis in non-nucleated structures.
La apoptosis es un proceso normal durante la respuesta inmune. Los linfocitos T citolíticos emplean perforina y granzima B para eliminar a las células infectadas. La granzima B activa directamente las caspasas, pero también en otras vías como la activación de una molécula denominada Bid que actúa sobre la mitocondria favoreciendo la salida de citocromo C y activando la vía interna de la apoptosis. Pero además, La apoptosis juega un papel importante mediante la eliminación de los linfocitos B y T una vez que la respuesta inmune ha terminado. Esta acción está mediada por Bcl-2 y por la activadad antigénica. Se ha propuesto que las células altamente estimuladas por los antígenos, es decir que han desarrollado anticuerpos contra ellos, disminuyen disminuyen la influencia de Bcl-2 y aumentan la de los receptores de muerte de manera que son células más sensibles a sufrir apoptosis.
There are many sources that produce cellular stress, which may generate uncontrolled and wrong functioning of the cell. Cellular stress may be produced by DNA damage, division cell flaws, misfolded or altered proteins, increase of reactive molecules, and pathogen infections. If a threshold is crossed, all of them may start apoptosis.
Cancer is an example where apoptosis plays an important role. More precisely, the inhibition of apoptosis favors proliferation and progression of tumors. Cancer cell avoids apoptosis by mutations in the pro-apoptotic genes. For instance, mutations in the FAS receptor genes may lead to a low number of FAS receptors or defective receptors so that cancer cells can not be recognized by cytotoxic lymphocytes. Another mutations affect the expression of bcl-2 pro-apoptotic genes.
During aging, apoptosis is altered in different tissues. In some of them apoptosis is increases, but it is minimized in others. For example, the immune system, skeletal muscular tissue, cardiac muscle, and neurodegenerative pathologies show an increase in the apoptotic processes. Senescent cells, and cancer cells, are more resistant to apoptosis, that is why they are more easily observed during aging.
Bibliography
Elmore S. 2007. Apoptosis: A Review of Programmed Cell Death. Toxicology and pathology. 35:495-516.
Henson PM, Hume DA. 2006. Apoptotic cell removal in development and tissue homeostasis. Trends in immunology. 27:444-250.
Suzane M, Steller H. 2013. Shaping organisms with apoptosis. Cell death and differentiation. 20:669-675.