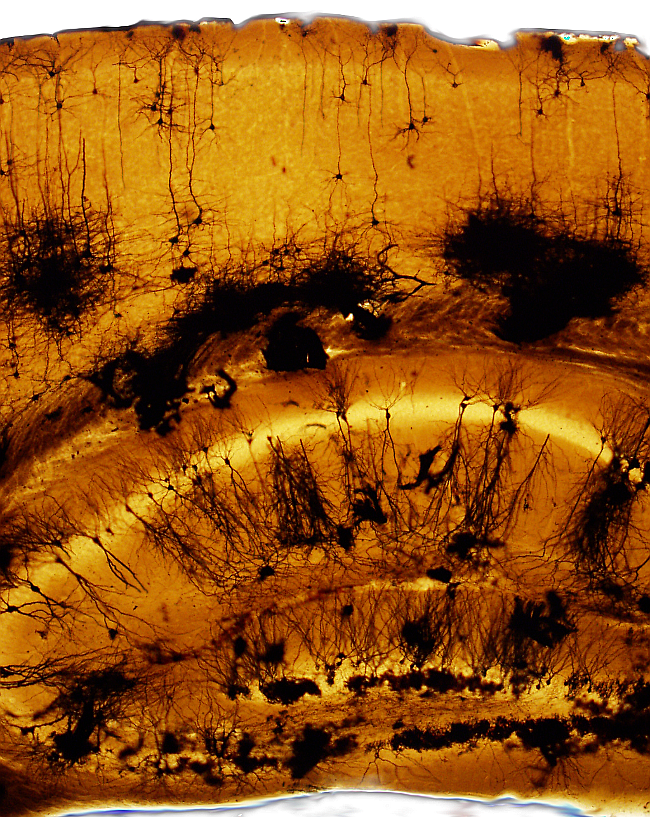
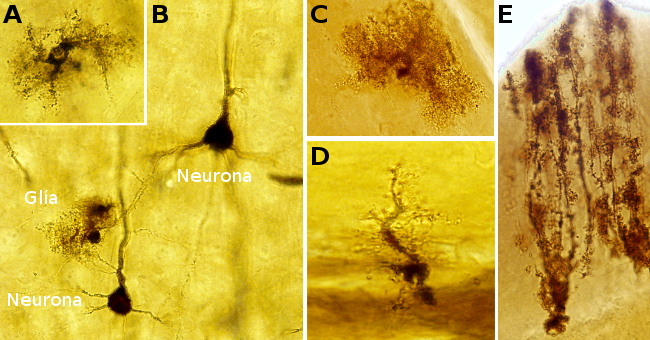
Visualizing neurons is complicated because their dendritic trees and long and branched axons occupy large portions of tissue that exceed the thickness of common sections. The Golgi technique is a silver impregnation (no staining) allows studying neurons: soma, dendrites, and axons. This technique label only a few neurons of the nervous tissue, so that the morphology of individual neurons can be studied. Glia can also be impregnated. The chemical reaction and why just a few cells are labelled are unknown. This technique allowed to visualize complete neurons for the first time.
La técnica de Golgi se realiza habitualmente en bloques de tejido nervioso (0,5 a 1 mm). El siguiente protocolo describe la adaptación de esta técnica a secciones gruesas.
Procedure
A brain, or a piece of brain, is fixed in buffered 4 % paraformaldehyde. Then it is sectioned with a vibratome into 100 to 300 µm thick sections. Sections are transferred to a buffered solution until they are processed. They can be stored for days.
1. 10 min in 1 % osmium tetroxide in phosphate buffer (0.1 M, pH 7.3)
Osmium tetroxide is highly toxic and volatile. Thus, it must be handled with gloves, in a fume chamber, and inactivated with corn oil.
2. 2 x 10 min in phosphate buffer.
3. 2 - 3 h in 3.5 % potassium dichromate in distilled H2O
4. Move and extend the sections onto a slide. Handle with a small brush
5. Remove the excess of potassium dichromate with an absorbent paper. Section must not get dry.
6. Place a coverslip onto the sections, and remove the air bubbles between the section and the coverslip.
7. Add 1.5 % silver nitrate dissolved in distilled H2O between the slide and the coverslip, drop by drop. The silver nitrate solution is extended by capillarity touching the edges of the section, but not the cut surfaces.
The solution can be added with a micropipet
8. Place the "sandwich" (slide, section, coverslip) in a humid chamber until the neurons are impregnated. impregnadas.
The time to get a good impregnation may vary between sections. It may last from hours to days. Anyway, the progress of the impregnation can be checked under the microscope.
9- The coverslip is removed, and the sections are quickly transferred to distilled H2O. With a small brush, the sections are stirred for a deep wash.
10. Quick dehydration. A few seconds in 50º, 80º 96º y 100º ethanol.
To maintain a strong and long-lasting impregnation, dehydration must be quick, although water needs to be removed. After that, the sections are embedded in epoxy-like resins.
11- 2x5 min in propilene oxide
12- Epoxy-like resin overnight
13. 48 h polymerization at 60 ºC.
The free-floating sections are placed onto slides and a drop of resin is added, and a coverslip is placed onto the sections. It is good practice to put some weight onto the coverslip for ironing the sections.
Resultados
Neurons: dark brown, black.
Astrocytes: dark brown, black.
Background: light brown, light orange.
Blood vessels: dark.
Notes
The number and how fast the neurons are impregnated may depend on the osmium tetroxide incubation time.
The silver nitrate solution must be completely clear. If it turns turbid, it must be discarded.
The impregnation is lost or decreased when the time in dehydration ethanol steps is long (more than 2 or 3 min).
The conventional mounting media used after xylene in common staining protocols do not keep neurons dark for a long time, and the impregnation is progressively lost.
Products
50º, 70º, 80º, 90º, 96º y 100º ethanol
Osmium tetroxide
Potassium dichromate
Silver nitrate
Epoxy-like resin
Distilled H2O
Labware
Humid chamber
Coverslip
Absorbent paper
Small brushes
60 ºC stove
Corn oil