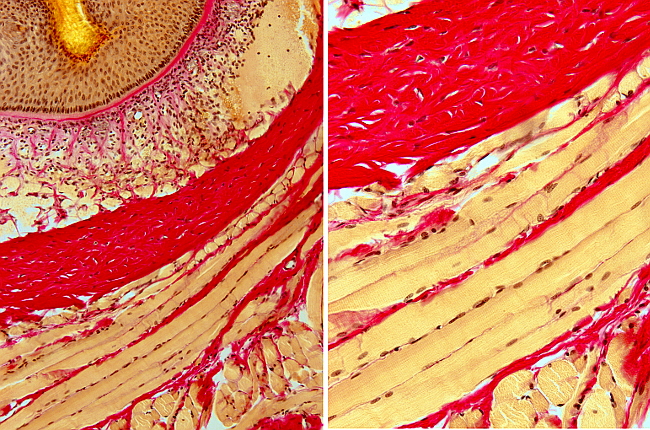
This staining protocol combines a nuclear staining with Weigert hematoxylin and a picric acid/acid fuchsin solution, which stains collagen fibers of the connective tissue.
Procedure
Whatever common fixative for general stainings is suitable for this protocol. Samples are embedded in paraffin and 8 µm thick sections are obtained and attached to gelatin coated slides.
1. 2x10 min in xylene
2. 2x10 min in 100º ethanol
3.- 10 min in 96º ethanol
4. 10 min in 80º ethanol
5. 10 min in 50º ethanol
6. 5 min in distilled H2O
7. 10 min in Weigert hematoxylin. This staining solution is composed of two separate solutions that are mixed immediately before use.
8. 5 min in tap water
9. 1 min in distilled H2O
10. 4 - 5 min in Van Gieson's pricrofuchsin
Van Gieson's picrofuchsin
Solution A. 1 % Acid fuchsin.
1 g acid fuchsin (C.I. 75290)
100 ml distilled H2O
Solution B. Saturated picric acid solution.
Working solution.
1 ml of solution A
45 ml of solution B
The working solution can be made just before the staining, although several weeks old solution can also be used by adjusting the staining time. Anyway, 0.25 ml of hydrochloric acid is added before use. Instead of just water, the following acidified water step is recommended to preserve a strong staining.
11. 2 x 30 s in acidified H2O
Acidified H2O:
0.5 ml of glacial acetic acid
100 ml of distilled H2O
12. 3 x 1 min in 100º ethanol
13. 2 x 5 min in xylene
14. Mounting media
Resultads
Collagen: red - pink
Muscle: yellow-orange
cytoplasm: yellow - orange
Nuclei: blue -black
Labware
Xylene
50º, 70º, 80º, 90º, 96º y 100º ethanol
Weigert hematoxylin
Acid fuchsin (C.I. 75290)
Picric acid
Galical acetic acid
Hydrochloric acid
Distilled H2O
Tap water
Mounting medium
Labware
Staining dishes
Slides racks
Coverslips