Cells adhere to other cells and to the extracellular matrix by means of adhesion transmembrane proteins. Integrins, cadherins, selectins and immunoglobulins stand out. Sometimes many adhesion proteins join to each other, and to other proteins, to form large macromolecular adhesion complexes, generally known as cell junctions. These molecular scaffolds are essential to maintain the integrity of many tissues, such as epithelial, muscular, and nervous tissues.
Cell junctions are classified according to their morphology, the type of adhesion molecules they contain, the structures they adhere to, and their interactions with the cytoskeleton. Cell junctions were first observed with electron microscopes and were named according to their morphology, but it was the molecular biology techniques that uncover their complex molecular organization.
1. Tight junctions
Tight junctions (Figure 1), also known as zonula occludens, are found in the more apical part of the epithelial cells, in cardiac muscle, in the endothelium of the central nervous system, and hepatocytes. They make so strong and tight contacts between adjoining cells that the intercellular space is nearly sealed.
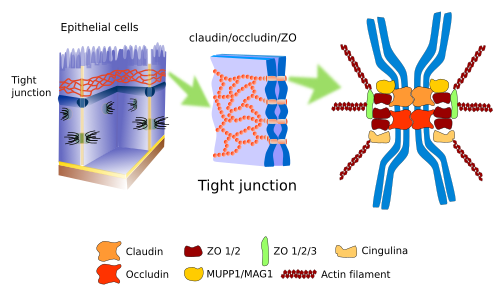
In the epithelial cells, tight junction work as a zipper around the cell perimeter. Apart from the strong adhesion, tight junctions carry out other functions. For example, in the intestine epithelium, tight junctions prevent molecules to diffuse through the intercellular space, between enterocytes, and are forced to cross through the enterocyte (transcellular pathway), a much more selective pathway. Furthermore, tight junctions maintain the polarity of epithelial cells because they form a physical barrier, so that they hamper lateral diffusion of membrane molecules, both proteins and lipids. It is a physical barrier for lateral diffusion in the plasma membrane. Thus, an apical plasma membrane domain is established with a particular set of molecules, different from the basolateral domain. This is essential to properly direct molecules from the intestine lumen toward the blood vessels: enter the enterocyte through the apical domain and exit through the basolateral domain.
In the nervous tissue capillaries, endothelial cells are strongly linked to one another by tight junctions, which help to form the blood-brain barrier. The blood-brain barrier is a very restrictive filter for the exchange of molecules between the blood and nervous cells (neurons and glia).
Tight junctions are composed of more than 40 types of proteins. Transmembrane proteins are the occludins, a family of proteins known as claudins, and the JAM proteins (Junctional Adhesion Molecules). Claudins are transmembrane proteins in charge of making the cell-cell adhesion, and between the intercellular adhesion points there are very narrow extracellular spaces (about 1 nm) that allow ions to travel through the extracellular space. There are twenty types of claudins that form passages with different sizes. Cells can change the expression of claudin types, thus regulating the permeability through the intercellular space. Occludins are not really necessary for tight junctions, but they provide stability to the cell junction and help to create a better barrier. JAM proteins also establish intercellular connections, but they appear to be more important in stabilizing the macromolecular scaffold. The intracellular domain of these proteins associates with other molecules known as ZO (zonula occludens), which in turn are connected to actin filaments and other cytosolic proteins. Depending on the adhesion state of the cell, these intracellular molecular interactions may trigger signaling pathways that affect the cell physiology. An interesting observation is that the occurrence of tight junctions in cells may need the presence of adherent junctions.
2. Adherent junctions
Adherent junctions (zonula adherens) are cell junctions that link epithelial cells. They are found in the apical part of the cell, just below the tight junctions (Figure 2). During development, adherent junctions are the first cell junction type to appear in epithelia, before tight junctions. They are thought to be involved in morphogenetic processes during embryo development. Like tight junctions, adherent junctions form a belt-like structure all around the cell, although they can also be found as small plates. The extracellular domains of E-Cadherins and nectins are involved in these cell-cell adhesion contacts. Their intracellular domains are linked to molecules like β- y α-catenins, catenin p120 and afadin. These proteins are intermediaries between the adhesion molecules and actin filaments. Furthermore, β-catenin can be released from the adhesion complex, enters the nucleus and changes gene expression.
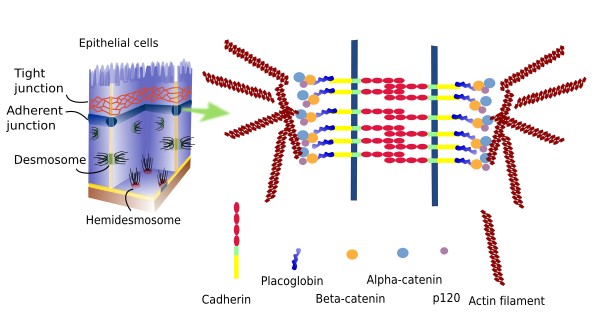
Adherent junctions are sequentially assembled. First, junctions mediated by nectins are established, which form rather weak adhesions, and then cadherins are recruited to form stronger and more stable contacts between cells. In some cellular types, adherent junctions facilitate the formation of tight junctions. Occludins, together with their protein scaffold, are first recruited by the adherent junctions, and ZO proteins may have a relevant role in this process.
Tight junctions, adherent junctions and desmosomes are needed for the integrity of the epithelial layer. However, only the adherent junctions are needed for the coordinated movement of groups of epithelial cells through the epithelial layer, which is a relatively frequent type of movement. Cell-cell adhesions made by adherent junctions build a connected network made up of actin filament bundles of different cells. These connected cells behave coordinately, for example, to cover a wounded region.
3. Desmosomes
Desmosomes (macula adherens) make spot cell-cell adhesions, like rivets (Figures 3 and 4). They are very abundant in epithelial and muscle tissue, but they can be found in virtually every tissue, for example in the nervous tissue. Cadherins (desmoglein and desmocollin) are the adhesion proteins in desmosomes. The intracellular domains of desmosome cadherins bind to intermediate filaments, such as keratin, through intermediary proteins.
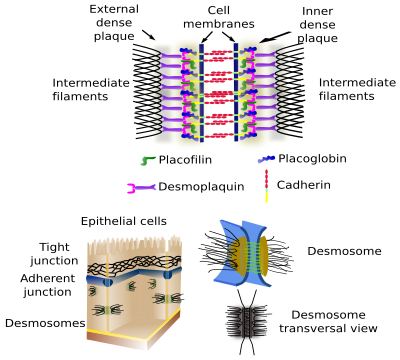

4. Hemidesmosomes
Hemidesmosomes (Figures 4 and 5) and focal adhesions make adhesion contacts between cells and the extracellular matrix. Both contain integrins as the adhesion molecule. Hemidesmosomes are junctions between epitelial cells and the basal lamina (a type of extracellular matrix). Intracellularly, the cystolic domain of these integrins are associated to intermediate filaments (Figure 5). Although hemidesmosomes look like one half of a desmosome, they are composed of different molecules. Focal adhesions are smaller and make junctions between cells and extracellular matrix by means of integrins, which are attached intracellularly to actin filaments.
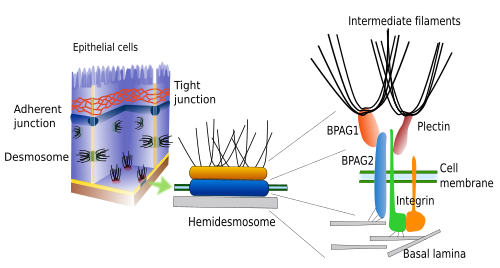
It is frequent to see gap junctions included as a member of the cell junction group. Gap junctions are molecular channels located in the plasma membrane that allow the direct communication between the cytoplasm of adjoining cells. That is why gap junctions should be regarded as cell-cell communication molecular complexes instead of involved in cell-cell adhesion.
-
Bibliografía ↷
-
Bibliografía
Campbell HK, Maiers JL, DeMali KA. (2017). Interplay between tight junctions and adherens junctions. Experimental cell research 358: 39-44.
Hahn B-S, Labouesse M. (2001). Tissue integrity: Hemidesmosomes and resistance to stress. Current biology 11:R858-R861.
Huber O. (2003). Structure and function of desmosomal proteins and their role in development and disease. Cell and molecular life science. 60:1872-1890.
Ladoux B, Mege RM. (2017). Mechanobiology of collective cell behaviours. Nature reviews in molecular cell biology. 18:743-757.
Niessen CM. (2007). Tight junctions/adherens junctions: basic structure and function. Journal of investigative dermatology. 127:2525-2532.
-
 Adhesion
Adhesion 