Proteins are responsible for many of the functions carried out by membranes. The protein-lipid ratio may vary in different membranes, but it is roughly 1:40 in number of molecules and 40 % in weight, which means that membranes usually have a lot of proteins. The proportion of proteins may be even higher in those membranes with strong metabolic involvement, such as the inner membrane of mitochondria. About 1/3 of our genes code for membrane proteins.
Over the time, models depicting the molecular organization of cell membranes have been modified to reflect the importance of proteins as essential elements for the membrane structure and function. The current membrane model to build from is the fluid mosaic by Singer and Nicholson. In this model, proteins are randomly scattered in the membrane and can move freely. However, nowadays, the interactions of proteins with both intra- and extra-membrane molecules that restrict the lateral movements have been included in the model. There are many evidences suggesting that proteins are segregated in lateral microdomains, which depends on the membrane and the protein type. Furthermore, data from atomic force microscopes suggest changes in the models to include the proportion of proteins and the physical space they occupy (Figure 1).
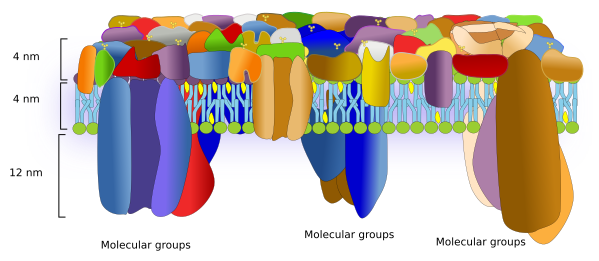
Proteins are numerous, diverse, and differentially distributed through the cell membranes. They can be classified in different ways. Regarding the function, they can be receptors, enzymes, adhesion molecules, channels, transporters, pumps, membrane handlers, and many others. Some proteins may be included in more than one category. According to how proteins are arranged in membranes, there are two main types: integral and associated proteins..
1. Integral proteins
Integral proteins are permanent components of the membrane. They may be transmembrane proteins, proteins spanning just one hemilayer, or proteins that are chemically linked to a membrane molecule.
Transmembrane
Transmembrane proteins show three moleculardomains: two hydrophilic and one hydrophobic (Figure 2). For instance, the transmembrane proteins of the plasma membrane proteins have an extracellular, an intra-membrane and a cytosolic domain. The intra-membrane domain contains sequences of hydrophobic amino acids that are located among the fatty acid chains of the membrane lipids, whereas the extra and intracellular domains contain hydrophilic amino acids. There are transmembrane proteins with only one hydrophobic domain, whereas others may have up to seven. Transmembrane proteins are mostly synthesized in the endoplasmic reticulum and distributed to other cell membranes by vesicular trafficking. Although transmembrane proteins may wrok alonde, many otransmebrane proteins need to be associated to other proteins to perform their functions, working as oligomeric groups.
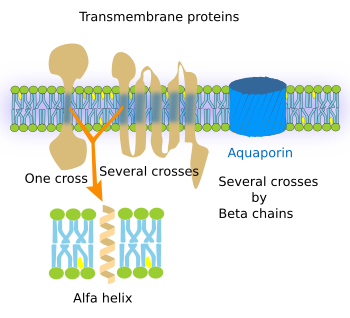
The functions of transmembrane proteins are diverse. a) Integrins, cadherins, and selectins are adhesion proteins. b) Pumps and ion channels generate and modify ion gradients between both sides of the membrane. For example, to produce ATP, or to exchange ions, such as calcium, sodium, and potassium. c) Transporters make possible large molecules to cross the membrane. For instance, they allow glucose to get into the cell by crossing the plasma membrane. d) Receptors enable cell communication by binding specific ligands, such as hormones, growth factors, neurotransmitters, and others, and transducing these signals into intracellular downstream molecular events. This can be done because transmembrane proteins have two hydrophilic domains, located at either sides of the membrane, which are connected by one or several intramembrane domains. After the recognition of an extracellular signal, a molecular rearrangement occurs in the transmembrane protein that is transmitted to the intracellular domain, which in turn causes a cytosolic molecular process, sometimes ending up with changes in gene expression. e) Some receptors and other transmembrane proteins show enzymatic activity in one of their hydrophilic domains.
Included or linked to one hemilayer
There are integral proteins that have part of the amino acid sequence spanning only one of the hemilayers of the membrane. Thus, they have one intramembrane domain and one external domain (Figure 3, on the left). If they are located in the plasma membrane, the external may be either cytosolic or extracellular.
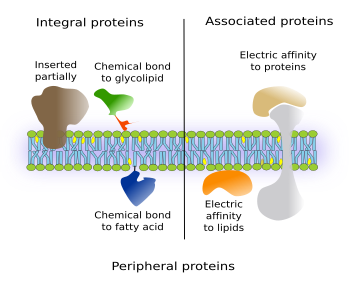
Another type of integral proteins includes those chemically linked to other membrane molecules, mostly glycolipids or fatty acids inserted among the membrane lipids. The entire amino acid sequence of the protein is outside the membrane. Most commonly, they are bound to GPI (glycosylphosphatidylinositol). These proteins were discovered in the seventies of the 20th century, and hundreds of them have been found in all eukaryotic cells studied so far. For instance, they are enzymes, adhesion molecules, and components of cellular coats in protozoa. It is interesting that in some cases, like the adhesion protein N-CAM, the same protein may be transmembrane or GPI-linked protein depending on mRNA alternative splicing.
The non-transmembrane proteins are also known as peripheral proteins, because they are related to only one hemilayer. Peripheral proteins also include other proteins referred to as associated protein (see below).
2. Associated proteins
Associated proteins are not permanently linked to membranes, i.e. they are not integral proteins. Instead of chemical bonds, electrical interactions, such as van der Waals forces, keep these proteins anchored to the hydrophilic surfaces of membranes (on the right in the Figure 3). These forces are weak, and proteins may switch between being attached to or detached from membranes. Associated proteins are hydrosoluble. For instance, G proteins are associated to the cytosolic surface of the plasma membrane.
-
Bibliografía ↷
-
Bibliografía
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., Walter, P. 2015. Molecular biology of the cell. Garlan Science (6ª edición). New York.
Pollard, T.D., Earnshaw, W.C., Lippincott-Schwartz, J., Johnson G. 2017. Cell biology. Saunders, Elsevier (3ª Edición). Philadelphia.
Zhao W, Tian Y, Cai M, Wang F, Wu J, Gao J, Liu S, Jiang J, Jiang S, Wang H. 2014. Studying the nucleated mammalian cell membrane by single molecule approaches. PLOSone. 9 (5):e91595.
-
 Lipids
Lipids