1. Structure
2. Nucleation
3. Organization
4. Miosin
5. Functions
- Cell shape
- Cell movement
- Internal organization
- Muscle contraction
- Cytokinesis
- Microvilli
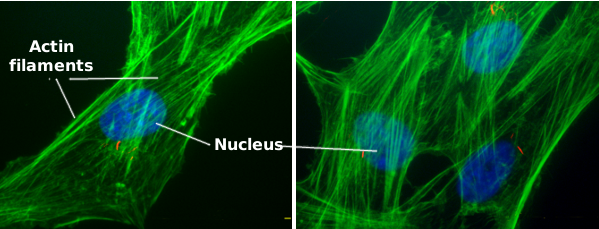
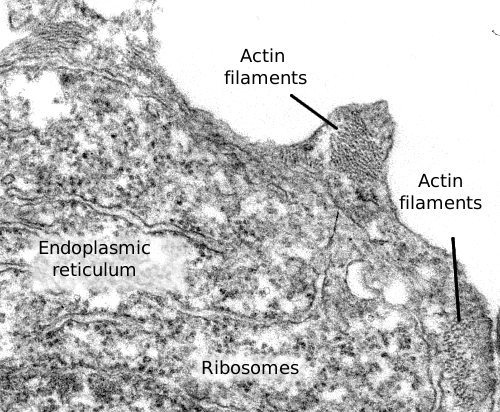
Actin filaments are a component of the cytoskeleton. In animal cells, actin filaments are usually found near the plasma membrane, where they form a cortical scaffold (Figures 1, 2 and 3) that provides mechanical support to the plasma membrane and to the cell too. However, their distribution and organization depend much on the cellular type. For instance, in plant and fungi cells, they show a different organization because the support role is acomplished by the cell wall.
Actin filaments perform many functions in the cell, such as cell division, cell movement, endocytosis, phagocytosis, and organelle communication. In animal cells, actin filaments provide mechanical support for maintaining or changing the cellular shape and are fundamental elements in cell muscle contraction that allow animal movement. Much aknowledge about actin filament behaviour has been gained from how pathogens invade cells. Studying these pathogens and their mutants has been helpfull in understanding the functional aspects of actin filaments.
1. Structure
Actin filaments are built by polymerization of free globular actin proteins (Figure 4), which can be found in two isoforms: alpha- and beta-actin. Alpha-actin is abundant in muscle cells. Beta-actin is the most frequent isoform, and it is found in most animal cells. Actin is a very abundant protein in the cytosol, accounting for about 10 % of the total cytosolic protein content. In the total cytosolic actin protein pool, some are found as part of the actin filaments (known as F-actin), and the remaining are free in the cytosol (known as G-actin). The proportions of both types change according to the cell needs, that is, the number and length of actin filaments after polymerization and depolymerization processes.
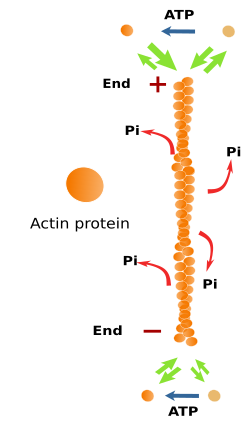
Actin filaments are 7 nm thick, thinner than the other two cytoskeleton components:microtubules and intermediate filaments. That is why actin filaments are also known as microfilaments. Actin filaments have a minus end and a plus end, which means that they are polarized filaments. It is a consequence of the ordered disposition of actin proteins along the filament. At the plus end, polymerization, the addition of new actin proteins, is more frequent than depolymerization, whereas at the minus end, depolymerization is more frequent. The increase and decrease in the microfilament length are caused by polymerization and depolymerization, respectively. In the cell, these changes are happening all the time, and they depend on associated proteins (see below). Actin filaments are the most dynamic components of the cytoskeleton, much more than microtubules and intermediate filaments.
2. Nucleation
Most free actin proteins are associated with profilin proteins; that is, the amount of "naked" actin in the cytosol is very low. The cytosolic environment and the concentration of non-associated actin proteins in the cytosol prevent the spontaneous assembling of actin filaments. Some pairs and triplets of actin proteins may be formed, but they are very unstable and quickly disgregated. Hence, the formation of new microfilaments relies on nucleation protein complexes, such as Arp2/3 and formins, and other proteins with domains to link actin proteins, such as Spire, Cobl and Lemoidin. Arp2/3 proteins work as nucleation templates for new microfilaments. First, they need to be activated and associted with the lateral part of other existing actin filaments. From this point, a new actin filament is formed with a angle of about 70°. Formins stabilize transient spontaneous associations of actin proteins, boosting the formation and elongation rate of microfilaments. This control of microfilament formation by the different nucleation proteins is very useful for the cell because new microfilaments are formed when and where they are needed by precisely placing nucleation protein complexes. For instance, the actin filaments that form the constriction ring during cytokinesis are assembled by formins.
3. Organization
A salient feature of actin filaments is that they are highly adaptable. They are formed and removed easily, and are associated with each other in many ways to form 3D scaffolds. This versatility relies on more than 100 different modulator protein types, or actin associated proteins (Figure 5). It can be said that these proteins use actin as a building material. Actin associated proteins regulate filament polymerization and depolymerization rates, the nucleation of new filaments, the destruction of existing filaments, and 3D organization. Actually, there are no "naked" actin filaments or free actin proteins in the cytosol, but they are always linked to some associated protein.
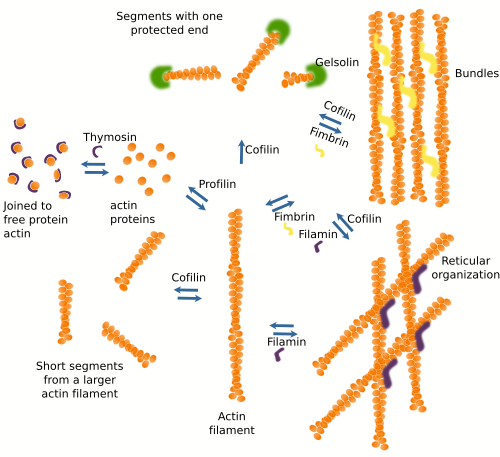
Proteins associated with actin filaments can be divided according to their functions. a) Modulate polymerization. Some assoaciated proteins favor actin filament polymerization, whereas others promote depolymerization. Profilin increases polymerization at the plus end of the actin filament but not at the minus end. Thymosin joins free actin proteins and hampers the polymerization process by preventing the spontaneous polymerization of actin filaments. Arp2/3 and formin promote nucleation. b) The organization of actin filaments areis also modulated by actin associated proteins. Fimbrin and alpha-actinin establish cross-bridges between actin filaments to form bundles, whereas other proteins, like filamin, lead to reticular structures. Each of these proteins builds actin filament scaffold with different properties. For instance, alpha-actinin leads to actin filament bundles that withstand occasional mechanical forces and allow cell movements when these forces are weak and long-standing. c) Some actin binding proteins, such as cofilin, severin, and gelsolin, break and rearrange the actin filaments. Profilin, however, stabilizes actin filaments acting at the menus end and protein caps at the plus end. d) There are also proteins that act as intermediaries between actin filaments and other proteins. For instance, tropomyosin mediates the interaction between actin filaments and myosin in the muscle cells. e) Some proteins are in charge of anchoring actin filaments to other cellular structures like adhesion complexes, the plasma membrane and other inner membranes. Some actin associated proteins may perform more than one function. For instance, formin allows the nucleation of new actin as well as actin filament polymerization by attracting profilin-actin to the plus end. In addition, profilin participates in the formation of cross-bundles of actin filaments.
There are additional elements modulating the effect of actin associated proteins on actin filament behavior, such as calcium concentration, activity of Rho-GTPases, lipids, and higher or lower gene expression for these proteins. There are also drugs that influence the polymerization of actin filaments. For example, cytochalasin inhibits polymerization, whereas phalloidin inhibits depolymerization.
4. Myosin
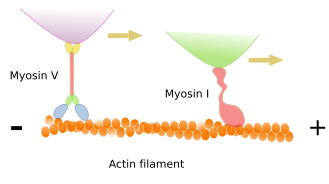
Many functions performed by actin filaments dependen on the type of motor proteins known as myosins. These proteins can move cargoes along the actin filament toward the plus end. The energy comes from ATP hydrolysis. Besides dragging cargoes, myosins can also slide two actin filaments, one over the other, in opposite directions. Furthermore, if the myosin is anchored, then the motor traction can move the actin filament. Myosins are actually a diverse family of proteins with more than 40 members in mammals: myosin I, myosin II, myosin III, and so on. Myosin I contains a molecular domain known as the head that links actin filaments and a tail to link cargoes to be dragged. Since myosin I only has one head, two or more myosin I molecules must join to be able to "walk" along the actin filament. Myosin I is found in most cells and is involved in the inner movement of cell compartments. During evolution, myosin I appeared before myosin II. Myosin II is mainly found in muscle cells, although it is also expressed in other cell types. Myosin II molecules associtated with one another to form filaments or structures resembling a segment with two arrowheads, where the arrowheads are the globular part of the myosin II molecules. In this way, these myosin filaments can drag cargoes (usually actin filaments) to the middle region of the filament. It is the mechanism in mucle cells during contraction. Myosin II is also involved in the strangling of cell compartments (see below). Myosin V and VI transport cargoes along the actin filaments. Myosin VII and X allow the protrusion of the cell surface to form cell extensions (see below).
5. Functions
Cell shape
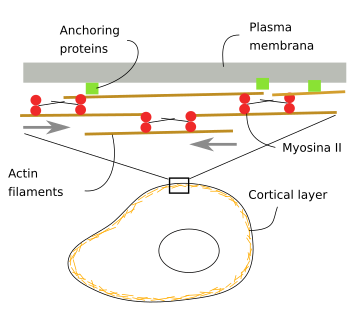
Underneath the plasma membrane, there is a cortical layer of actin filaments about 100 nm thick (Figure 6). They are interlocked to each other by associated proteins and anchored to proteins and lipids in the plasma membrane. This cortical layer is thicker in differentiated cells that in proliferating cells. It also contains myosins that generate mechanical forces that push actin filaments and change the position of the plasma membrane. This actin filament layer makes cells both withstand and generate mechanical forces, influencing, in this way, the cell shape. The organization of actin filaments may change according to the type and direction of the mechanical stress, the cell type and even the cell surface region. For instance, it cannot be observed at some points of the cell surface. Animal cells lack a cell wall, so cell shape largely depends on the actin filaments of the cortical cytoplasm, both those found in the cell cortical region and those connecting with adhesion proteins and cell adhesion complexes.
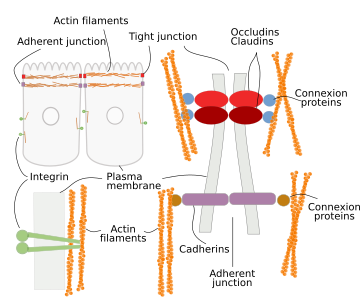
In animal cells, the cell shape largely depends on the adhesion contacts with the extracellular matrix and other adjoining cells (Figure 7). Integrins mediate the adhesion of the cell to the extracellular matrix. In their cytosolic domain, integrins are connected to actin filaments, so there is mechanical continuity between the extracellular matrix and cytoskeleton. Tight junctions and adherent junctions are cell-cell junctions made up of adhesion transmembrane proteins, occludin/claudin and cadherin, respectively, which are also connected to actin filaments by intermediary proteins. Thus, the scaffold of actin filaments is a mechanical sensor of the tension forces acting on the cells. This is clear in epithelial cells
Cell movement
Cells do not swim, but they crawl or trail across the tissues, as do, for example, the embryonic cells during development, amoebas when moving around, lymphocytes when heading toward damaged tissues, and the growth cone of neurons when searching for their targets. For cell movement, several steps are needed: the production and extension of cytoplasmic protrusions, the adhesion of these protrusions to some elements of the environment, and the dragof the rest of the cell toward these anchoring points. The cellular protrusions are known as podia, and there are lamellipodia when they are sheet-like, filopodia when they are thin and long, and lobopodia when they are thick and tubular. Actin filaments have a prominent role in the formation of these protrusions (Figure 8), because after treatment with cytokalasin, a polymerization inhibitor, cells stop moving and the cell expansions disappear. Actually, actin polymerization is what pushes the plasma membrane outward and forms protrusions.
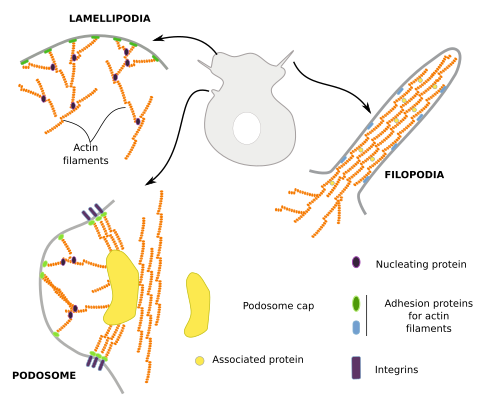
Lamellipodia are rather flat cell expansions originated by actin filament polymerization that, instead of bundles, form a branched scaffold. These expansions appear when cells are moving and during phagocytosis and macropinocytosis. Arp2/3 and formin proteins are needed to form lamellipodia. Filopodia may emerge from lamellipodia or from other cell regions. Typical filopodia are a few µm in diameter and no more than 10 µm in length. They contain dozens of actin filaments organized as a bundle. Podosomes are cell expansions attached to the extracellular matrix through integrins, but they are also able to release metalloproteinases for extracellular matrix degradation. Macrophages, dendritic cells, osteoclasts and endothelial cells develop podosomes. They contain a central branched scaffold of actin filaments surrounded by non-branched actin filaments. Podosomes may be mechanosensors that explore the environment and may also contribute to cell movement. Invadosomes are a particular type of cell expansion found in cancer cells. Invadosomes are larger and longer-lasting than podosomes.
When these cell expansions contact any extracellular structure, either extracellular matrix macromolecules or other cells, they get anchored by adhesion proteins like integrins and cadherins. Once anchored, the cell pulls all the intracellular compartments and structures toward the adhesion points. The dragging force relies on the stress fibers, intracellular structures composed of actin filaments and myosin proteins (Figure 9). Not all actin filaments are perfectly oriented in the stress fibers. Bundles of stress fibers form focal adhesions.
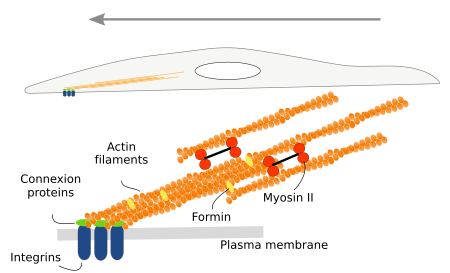
Internal organization
Actin filaments are mostly found near the plasma membrane, in the so-called cortical region. In this place, they are involved in vesicle formation, macropinocytosis and phagocytosis (Figure 10). The invagination and escission of vesicles are not accomplished if actin polymerization is inhibited. Cell extensions formed during macropinocytosis and phagocytosis need the polymerization of actin filaments.

Organelles are moved through the cytoplasm to perform their function or for cytoplasmic reorganization. Actin filaments, together with myosin motor proteins, help with the organelle movements (Figure 11). This role is very important in plant cells, where most of the internal movements of organelles are carried out by actin filaments. It has been observed for Golgi apparatus cisterns and chloroplasts. The chloroplast movement, a process known as cyclosis, can be observed under the light microscope. Vesicular trafficking is also mediated by actin filaments and myosin proteins. In animal cells, the organelle movements are mediated by both microtubules and actin filaments. There is another weird way of moving organelles by actin filaments: the plus end of a short actin filament joins an organelle, and it is the polymerization of the actin filament that propels the organelle through the cytoplasm.
Mitochondria need actin filaments for normal functioning because, together with the endoplasmic reticulum, actin filaments participate in mitochondrial fission.
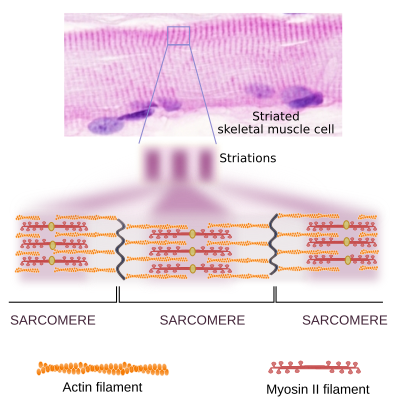
Muscle contraction
In muscle cells, myosin II molecules are associated with each other to form the thick myosin filaments, which show polarity as if they were an arrow with two arrowheads, each at one end (Figure 12). In the skeletal striated muscle, each of these heads drags actin filaments toward the middle point of the myosin filament. This is the molecular mechanism for muscle cell contraction. In smooth muscle cells, the actin and myosin filaments interact after the phospholytion of myosin II, which is a slower mechanism.
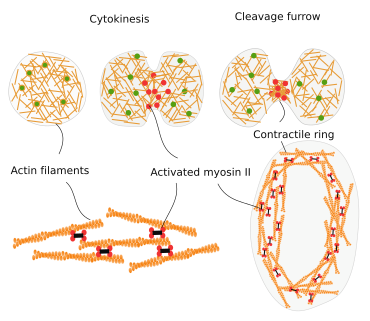
Cytokinesis
During cell division, the final strangling of the cytoplasm is produced by a ring of actin filaments that, helped by myosin II motor proteins, gets progressively smaller in diameter until the complete separation of the cytoplasm of the two new cells (Figure 13). Many other proteins are involved in the cytokinesis ring, which perform their roles sequentially. The nucleation of actin filaments begins in discrete regions known as nodes, composed of myosin II and formin. In the nodes, the nucleated actin filaments are aligned and interact with each other. Myosin II slides some actin filaments over the others, making a ring and making the diameter of this ring narrower. It is interesting that as the ring become narrower, the number of actin filaments involved decreases.
Microvilli
Microvilli are filiform expansions of the apical part of some cells that enormously increase the surface of the plasma membrane. For example, they can be found in the epithelium of the gut and in the proximal tube of the nephron. Each of these little expansions, or microvillus, is 1 to 2 µm heigh and around 0.1 µm thick. They contain several dozens of actin filaments oriented parallel to the longitudinal axis of the microvillus (Figure 14). These filaments are connected to one another by other proteins, sucha as myosin, fimbrin, and vilin. Thus, they are able to peform slight movements. At the base of microvilli, in the peripheral cytoplasm, there is a molecular scaffold known as the terminal web, which is composed of actin filaments and stabilizes the whole set of microvilli.

-
Bibliography ↷
-
Bibliography
Pollard TD. 2019. Cell motility and cytokinesis: from mysteries to molecular mechanisms in five decades. Annual review of cell and developmental biology. 35:1-28.
Rottner K, Faix J, Bogdan S, Linder S, Kerkhoff E. 2017. Actin assembly mechanisms at a glance. Journal of cell science. 130: 3427-3435.
Svitkina TM. 2018. Ultrastructure of the actin cytoskeleton. Current opinion in cell biology. 54:1-8.
-
 Cytoskeleton
Cytoskeleton